Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0883–0912) RA – Diagnosis, Manifestations, and Outcomes Poster II
0898: Risk of Aortic Stenosis and Undergoing Aortic Valve Replacement in Rheumatoid Arthritis: An Underrecognized Cardiovascular Complication
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.png)
Tate Johnson, MD
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Tate Johnson1, Yangyuna Yang2, Chetaj Mahabir2, Andrew Goldsweig2, Punyasha Roul3, Joshua Baker4, Brian Sauer5, Grant Cannon6, Ted Mikuls7 and Bryant England2, 1University of Nebraska Medical Center, Elkhorn, NE, 2University of Nebraska Medical Center, Omaha, NE, 3UNMC, Omaha, NE, 4University of Pennsylvania, Philadelphia, PA, 5Salt Lake City VA/University of Utah, Salt Lake City, UT, 6Retired, Salt Lake City, UT, 7Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Valvular carditis is reported as a rare extra-articular manifestation of rheumatoid arthritis (RA). In animal models with coexistent inflammatory arthritis, pro-inflammatory infiltrates of T cells and macrophages as well as autoantibodies have been demonstrated to induce inflammation of cardiac valves including the aortic valve. Aortic stenosis (AS) is the most common reason for valve replacement and the most frequent cause of valvular heart disease-related death in the U.S. However, the burden of AS in RA is poorly understood. We examined the rates of incident AS and the need for an aortic valve intervention in RA and matched non-RA patients.
Methods: We conducted a retrospective, matched cohort study in the Veterans Health Administration (VA) from 1/2000 to 12/2018. Among individuals without a history of AS or aortic valve intervention, RA patients (≥2 ICD codes for RA, rheumatologist diagnosis, and positive autoantibody or DMARD fill) were matched up to 1:10 on age, sex, and enrollment year to patients without RA. Using validated diagnostic codes, we identified incident AS upon the earliest fulfillment of an AS hospital discharge diagnostic code, two outpatient diagnoses plus at least one echocardiogram, aortic valve intervention, or death related to AS using linked National Death Index data. Surgical aortic valvular replacement (SAVR), transaortic valvular replacement (TAVR), and balloon aortic valvuloplasty (BAV) were identified using ICD-procedure and CPT codes. Patients were followed to incident AS, aortic valve intervention, death, or end of study period, censoring those who did not receive VA care for ≥365 days. Baseline covariates were obtained from national VA databases and included demographics, smoking status, body mass index, Rheumatic Disease Comorbidity Index, and health care utilization. Multivariable Cox regression, clustered by matched pairs, was used to examine the association of RA with AS outcomes.
Results: We matched 72,761 RA patients to 643,423 non-RA patients. Patients were predominantly male (88%) with a mean age of 63 years. Mean age of AS onset was 74 years in RA and non-RA. Over a mean follow-up of 9.5 years, 2,234 RA (IR 4.10 per 1000 person-years [PY]) and 9,319 non-RA (IR 2.17 per 1000 PY) patients developed AS (Table 1). Unadjusted incidence rates of aortic valve interventions were also higher in RA than non-RA. After multivariable adjustment, RA was associated with an increased risk of AS (adjusted hazard ratio [aHR] 1.51, 95% CI 1.44-1.59) and aortic valve interventions (aHR 1.40, 1.23-1.60), including SAVR (aHR 1.38, 1.19-1.59), TAVR (aHR 1.48, 1.05-2.10), and BAV (aHR 2.58, 1.22-5.44).
Conclusion: In this national, matched cohort study, RA patients had an approximately 50% increased risk of developing AS and were 1.4-fold more likely to undergo aortic valve intervention. These epidemiologic findings parallel the risk of inflammation-mediated valvular carditis in animal models, underscoring the need to identify RA-related risk factors that drive AS risk and evaluate whether current treatment strategies effectively mitigate the risk of AS progression in RA.
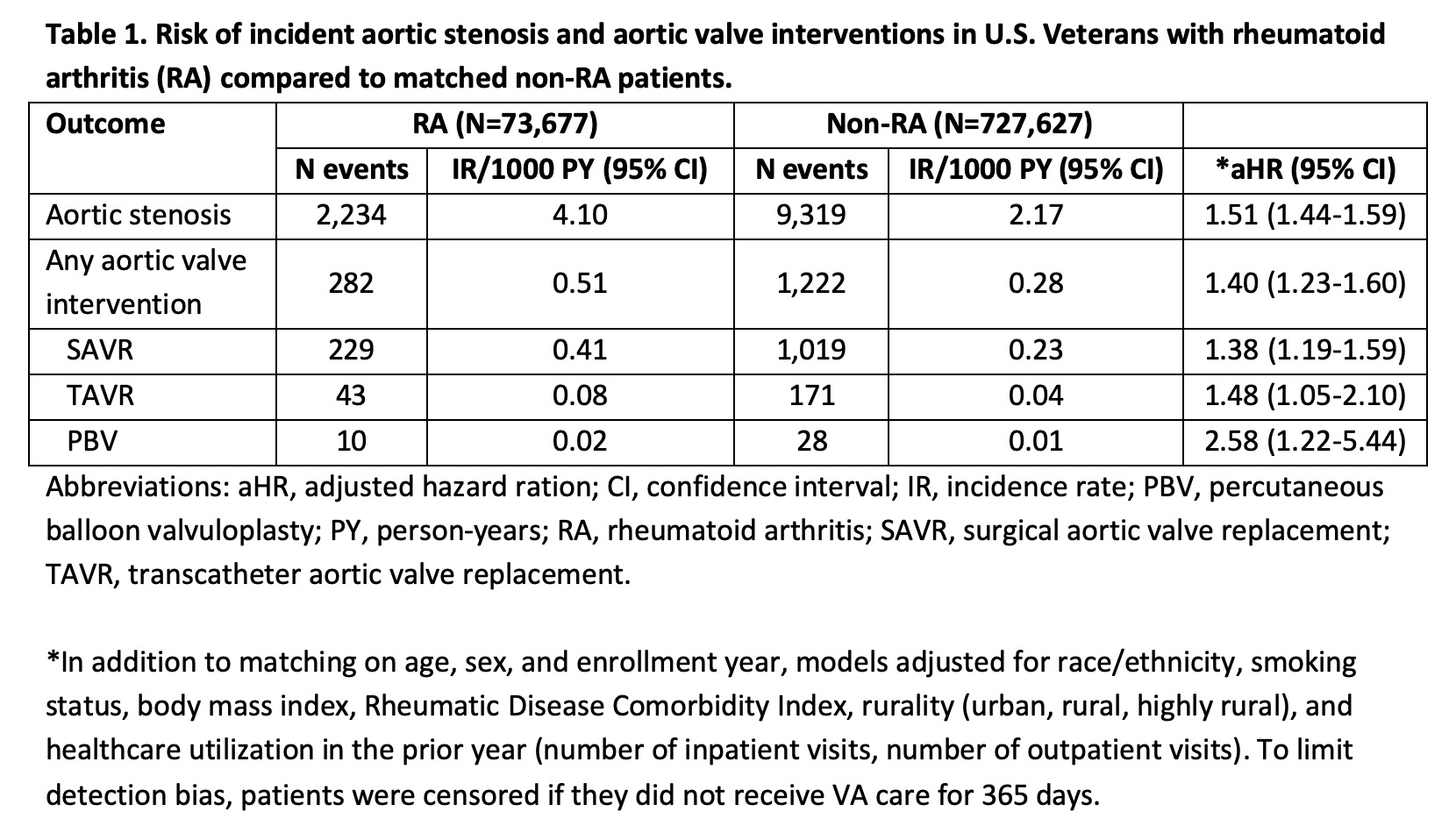 Table 1. Risk of incident aortic stenosis and aortic valve interventions in U.S. Veterans with rheumatoid arthritis (RA) compared to matched non-RA patients.
Table 1. Risk of incident aortic stenosis and aortic valve interventions in U.S. Veterans with rheumatoid arthritis (RA) compared to matched non-RA patients.
Disclosures: T. Johnson, None; Y. Yang, None; C. Mahabir, None; A. Goldsweig, Inari Medical; P. Roul, None; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; B. Sauer, None; G. Cannon, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; B. England, Boehringer-Ingelheim.
Background/Purpose: Valvular carditis is reported as a rare extra-articular manifestation of rheumatoid arthritis (RA). In animal models with coexistent inflammatory arthritis, pro-inflammatory infiltrates of T cells and macrophages as well as autoantibodies have been demonstrated to induce inflammation of cardiac valves including the aortic valve. Aortic stenosis (AS) is the most common reason for valve replacement and the most frequent cause of valvular heart disease-related death in the U.S. However, the burden of AS in RA is poorly understood. We examined the rates of incident AS and the need for an aortic valve intervention in RA and matched non-RA patients.
Methods: We conducted a retrospective, matched cohort study in the Veterans Health Administration (VA) from 1/2000 to 12/2018. Among individuals without a history of AS or aortic valve intervention, RA patients (≥2 ICD codes for RA, rheumatologist diagnosis, and positive autoantibody or DMARD fill) were matched up to 1:10 on age, sex, and enrollment year to patients without RA. Using validated diagnostic codes, we identified incident AS upon the earliest fulfillment of an AS hospital discharge diagnostic code, two outpatient diagnoses plus at least one echocardiogram, aortic valve intervention, or death related to AS using linked National Death Index data. Surgical aortic valvular replacement (SAVR), transaortic valvular replacement (TAVR), and balloon aortic valvuloplasty (BAV) were identified using ICD-procedure and CPT codes. Patients were followed to incident AS, aortic valve intervention, death, or end of study period, censoring those who did not receive VA care for ≥365 days. Baseline covariates were obtained from national VA databases and included demographics, smoking status, body mass index, Rheumatic Disease Comorbidity Index, and health care utilization. Multivariable Cox regression, clustered by matched pairs, was used to examine the association of RA with AS outcomes.
Results: We matched 72,761 RA patients to 643,423 non-RA patients. Patients were predominantly male (88%) with a mean age of 63 years. Mean age of AS onset was 74 years in RA and non-RA. Over a mean follow-up of 9.5 years, 2,234 RA (IR 4.10 per 1000 person-years [PY]) and 9,319 non-RA (IR 2.17 per 1000 PY) patients developed AS (Table 1). Unadjusted incidence rates of aortic valve interventions were also higher in RA than non-RA. After multivariable adjustment, RA was associated with an increased risk of AS (adjusted hazard ratio [aHR] 1.51, 95% CI 1.44-1.59) and aortic valve interventions (aHR 1.40, 1.23-1.60), including SAVR (aHR 1.38, 1.19-1.59), TAVR (aHR 1.48, 1.05-2.10), and BAV (aHR 2.58, 1.22-5.44).
Conclusion: In this national, matched cohort study, RA patients had an approximately 50% increased risk of developing AS and were 1.4-fold more likely to undergo aortic valve intervention. These epidemiologic findings parallel the risk of inflammation-mediated valvular carditis in animal models, underscoring the need to identify RA-related risk factors that drive AS risk and evaluate whether current treatment strategies effectively mitigate the risk of AS progression in RA.
 Table 1. Risk of incident aortic stenosis and aortic valve interventions in U.S. Veterans with rheumatoid arthritis (RA) compared to matched non-RA patients.
Table 1. Risk of incident aortic stenosis and aortic valve interventions in U.S. Veterans with rheumatoid arthritis (RA) compared to matched non-RA patients.Disclosures: T. Johnson, None; Y. Yang, None; C. Mahabir, None; A. Goldsweig, Inari Medical; P. Roul, None; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; B. Sauer, None; G. Cannon, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; B. England, Boehringer-Ingelheim.

