Back
Abstract Session
Session: Abstracts: Infection-related Rheumatic Disease (2272–2275)
2274: Factors Associated with Disease Flare Following SARS-CoV-2 Vaccination in People with Inflammatory Rheumatic and Musculoskeletal Diseases – Results from the Physician-Reported EULAR Coronavirus Vaccine (COVAX) Registry
Monday, November 14, 2022
5:30 PM – 5:40 PM Eastern Time
Location: Exhibit Hall A
- BF
Bayram Farisogullari, MD
Hacettepe University, Department of Internal Medicine Faculty of Medicine, Division of Rheumatology
Ankara, Turkey
Presenting Author(s)
Bayram Farisoğulları1, Saskia Lawson-Tovey2, Kimme Hyrich3, Laure Gossec4, Loreto Carmona5, Anja Strangfeld6, Elsa Mateus7, Martin Schaefer8, Ana Maria Rodrigues9, Eric Hachulla10, Jose A Gomez-Puerta11, Marta Mosca12, Patrick Durez13, Ludovic Trefond14, Tiphaine Goulenok15, Martina Cornalba16, Emoke Šteňová17, Inita Bulina18, Eva Strakova19, Julija Zepa20, Nicolas Roux21, Olivier Brocq22, Viellard Eric23, Bernd Raffeiner24, Gerd Burmester25, Xavier Mariette26 and Pedro Machado27, 1Hacettepe University Faculty of Medicine Division of Rheumatology, Ankara, Turkey, 2Centre for Genetics and Genomics Versus Arthritis, Centre for Musculoskeletal Research, the University of Manchester, Manchester, UK AND National Institute of Health Research Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, United Kingdom, 3The University of Manchester, Manchester, United Kingdom, 4Sorbonne Université, Paris, France, 5Instituto de Salud Musculoesquelética (InMusc), Madrid, Spain, 6Deutsches Rheuma-Forschungszentrum Berlin, Berlin, Germany, 7EULAR, Lisboa, Portugal, 8German Rheumatism Research Center, Berlin, Germany, 9Reuma.pt, Sociedade Portuguesa de Reumatologia, Lisbon, Portugal, 10University of Lille, LILLE, France, 11Hospital Clínic de Barcelona, Barcelona, Spain, 12Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 13Rheumatology, Cliniques Universitaires Saint-Luc – Université catholique de Louvain (UCLouvain) – Institut de Recherche Expérimentale et Clinique (IREC), Brussels, Belgium, 14Université Clermont Auvergne, CHU Clermont-Ferrand, Service de Médecine Interne, Hôpital Gabriel Montpied, INSERM U1071, Clermont-Ferrand, France, 15APHP, Paris, France, 16Dipartimento di Reumatologia e Scienze Mediche, ASST Gaetano Pini-CTO, Milano, Italy, 17University Hospital, Bratislava, Slovakia, 18Center of Rheumatology, Paul Stradins Clinical University hospital, Riga, Latvia, Riga, Latvia, 19Department of Internal Medicine, Faculty Hospital Prešov, Presov, Slovakia, 20Riga Stradins University, Latvia, Pauls Stradins Clinical University Hospital, Centre of Rheumatology, Riga, Latvia, Riga, Latvia, 21Service de Rhumatologie, Hôpital Robert Schuman, Metz, France, 22Rheumatology- CH Princesse Grace, Monaco, Monaco, 23Private practice, St. Malo, France, 24Department of Rheumatology, Central Hospital of Bolzano, Bolzano, Italy, 25Charité University Medicine Berlin, Berlin, Germany, 26Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 27University College London, London, United Kingdom
Background/Purpose: To investigate the frequency and factors associated with disease flare following vaccination against SARS-CoV-2 in people with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMD).
Methods: The European Alliance of Associations for Rheumatology (EULAR) Coronavirus Vaccine (COVAX) physician-reported registry is an observational registry of patients with a pre-existing inflammatory or non-inflammatory RMD who have received one or more doses of any vaccine against SARS-CoV-2. Four diagnostic groups were defined: (1) inflammatory joint diseases (IJD), (2) connective tissue diseases (CTD), (3) vasculitis, and (4) other I-RMD (OIRD). As disease activity was only collected at baseline, patients that received more than 2 vaccine doses were excluded from the analyses. Missing values for vaccine type and disease activity were derived by multiple imputation using full conditional specification. Predictors of flare were investigated using multivariable logistic regression adjusted for demographic and clinical factors. Two separate multivariable models were built, one using “disease flare” as dependent variable, and one using “new medication or dosage increase due to flare” as dependent variable.
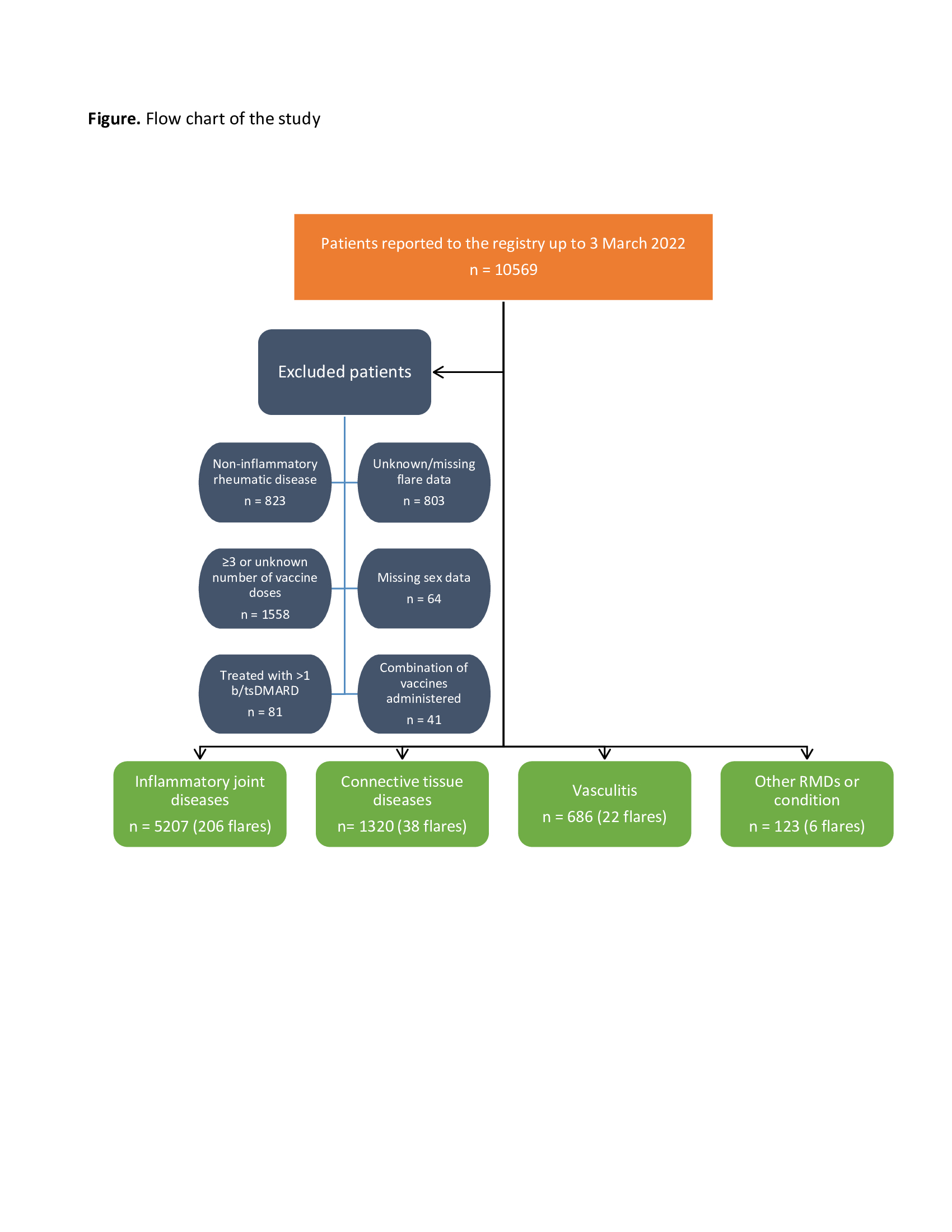
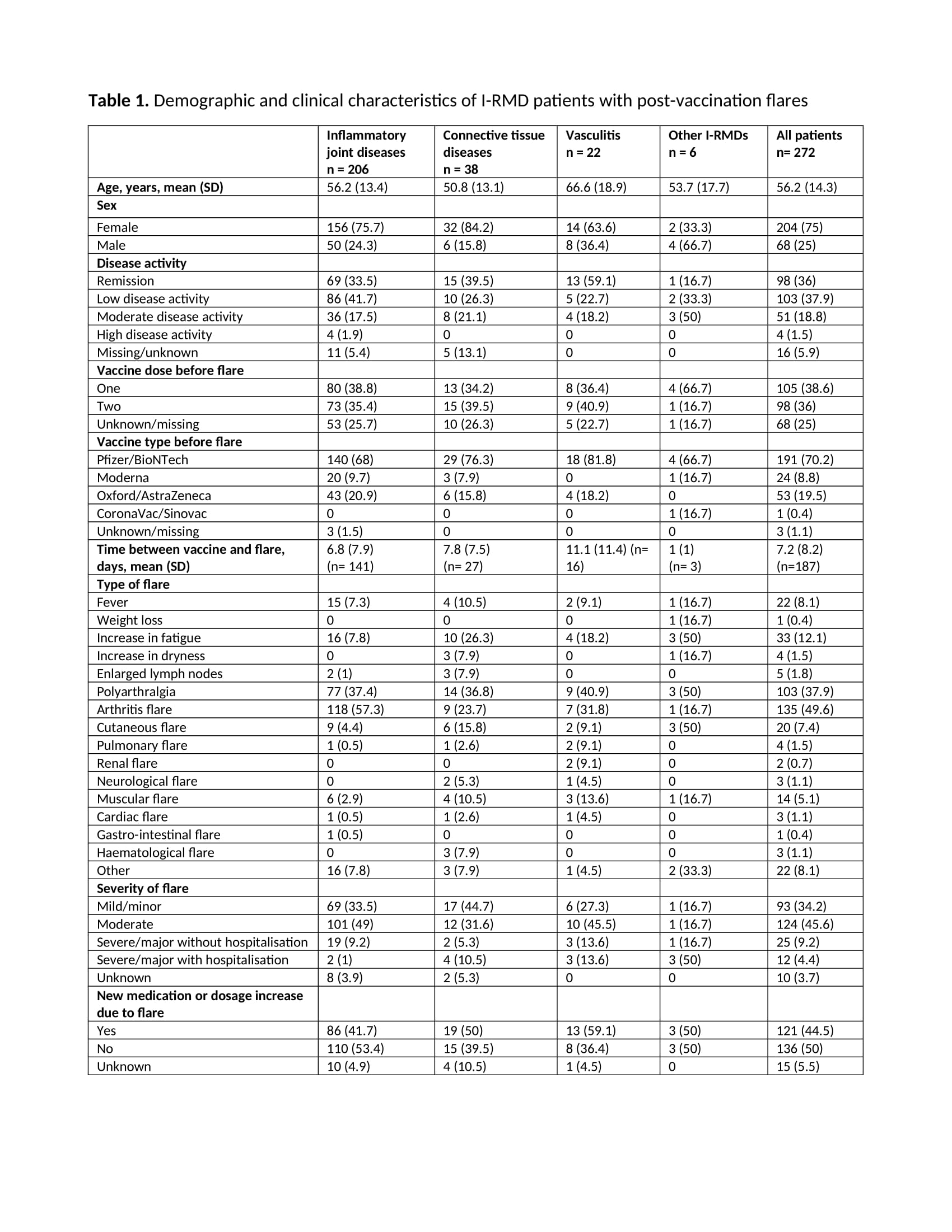
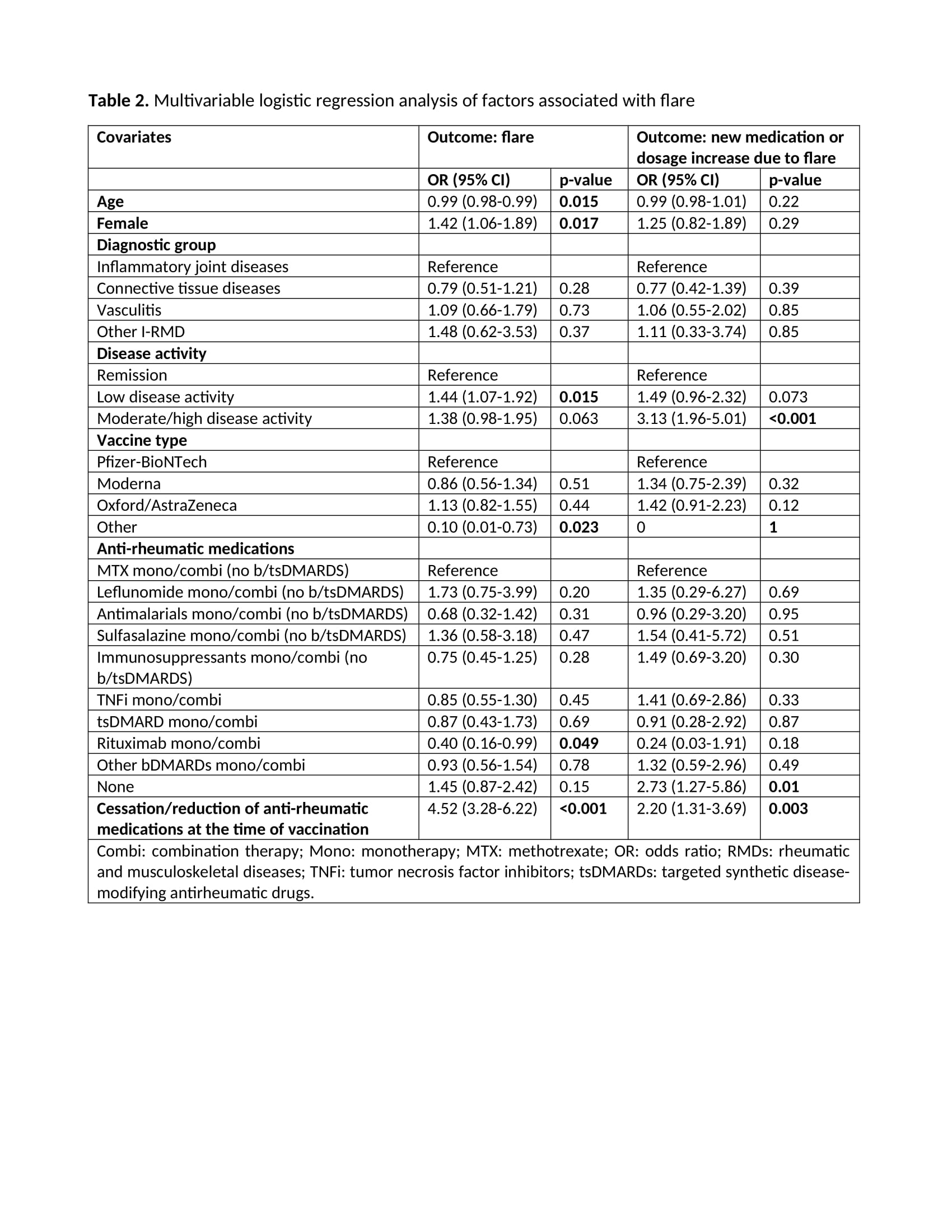
Results: Of 10569 patients reported to the registry by 3 March 2022, 7336 were included in this study (Figure). Most patients had IJD (71%), followed by CTD (18%), vasculitis (9.4%), and OIRD (1.7%). Disease flares were reported in 272/7336 (3.7%) patients (Table 1). Flares requiring starting a new medication or increasing the dosage of an existing medication were reported in 121/7336 (1.6%) patients. Mean time between flare and the most recent vaccine dose was 7.2 days (SD 8.2) (Table 1). Age (OR 0.99, 95%CI 0.98-0.99), female sex (OR 1.42, 95%CI 1.06-1.89), active disease (low disease activity (LDA), OR 1.44, 95%CI 1.07-1.92; moderate/high disease activity (M/HDA), OR 1.38, 95%CI 0.98-1.95; vs remission), other vaccine types different from Pfizer/BioNTech, Oxford/AstraZeneca and Moderna (OR 0.10, 95%CI 0.01-0.73; vs Pfizer/BioNTech), rituximab exposure (OR 0.40, 95%CI 0.16-0.99), and cessation/reduction of anti-rheumatic medication before or after vaccination (OR 4.52, 95%CI 3.28-6.22) were factors independently associated with disease flare (Table 2). In the model using new medication or dosage increase due to flare as dependent variable (Table 2), active disease (LDA, OR 1.49, 95%CI 0.96-2.32; M/HDA, OR 3.13, 95%CI 1.96-5.01; vs remission), not using anti-rheumatic medication (OR 2.73, 95%CI 1.27-5.86), and cessation/reduction of anti-rheumatic medication before or after vaccination (OR 2.20, 95%CI 1.31-3.69) were the only factors independently associated with this more strict flare definition.
Conclusion: Flares of underlying I-RMD following SARS-CoV-2 vaccination were uncommon. Factors associated with potential flares were identified, namely higher disease activity and cessation/reduction of anti-rheumatic medications before or after vaccination. These data will inform SARS-CoV-2 vaccination strategies in patients with I-RMDs.
Disclosures: B. Farisoğulları, None; S. Lawson-Tovey, None; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Carmona, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer; E. Mateus, None; M. Schaefer, None; A. Rodrigues, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; J. Gomez-Puerta, GSK, Galapagos, Pfizer, Janssen, Sanofi, AbbVie, Bristol Myers Squibb, Lilly, Novartis, MSD, Roche; M. Mosca, None; P. Durez, AbbVie, Galapagos, Lilly; L. Trefond, None; T. Goulenok, None; M. Cornalba, None; E. Šteňová, None; I. Bulina, AbbVie/Abbott, AstraZeneca, Janssen, Novartis; E. Strakova, None; J. Zepa, AbbVie/Abbott, Novartis, Janssen, AstraZeneca; N. Roux, None; O. Brocq, None; V. Eric, None; B. Raffeiner, None; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.
Background/Purpose: To investigate the frequency and factors associated with disease flare following vaccination against SARS-CoV-2 in people with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMD).
Methods: The European Alliance of Associations for Rheumatology (EULAR) Coronavirus Vaccine (COVAX) physician-reported registry is an observational registry of patients with a pre-existing inflammatory or non-inflammatory RMD who have received one or more doses of any vaccine against SARS-CoV-2. Four diagnostic groups were defined: (1) inflammatory joint diseases (IJD), (2) connective tissue diseases (CTD), (3) vasculitis, and (4) other I-RMD (OIRD). As disease activity was only collected at baseline, patients that received more than 2 vaccine doses were excluded from the analyses. Missing values for vaccine type and disease activity were derived by multiple imputation using full conditional specification. Predictors of flare were investigated using multivariable logistic regression adjusted for demographic and clinical factors. Two separate multivariable models were built, one using “disease flare” as dependent variable, and one using “new medication or dosage increase due to flare” as dependent variable.
Results: Of 10569 patients reported to the registry by 3 March 2022, 7336 were included in this study (Figure). Most patients had IJD (71%), followed by CTD (18%), vasculitis (9.4%), and OIRD (1.7%). Disease flares were reported in 272/7336 (3.7%) patients (Table 1). Flares requiring starting a new medication or increasing the dosage of an existing medication were reported in 121/7336 (1.6%) patients. Mean time between flare and the most recent vaccine dose was 7.2 days (SD 8.2) (Table 1). Age (OR 0.99, 95%CI 0.98-0.99), female sex (OR 1.42, 95%CI 1.06-1.89), active disease (low disease activity (LDA), OR 1.44, 95%CI 1.07-1.92; moderate/high disease activity (M/HDA), OR 1.38, 95%CI 0.98-1.95; vs remission), other vaccine types different from Pfizer/BioNTech, Oxford/AstraZeneca and Moderna (OR 0.10, 95%CI 0.01-0.73; vs Pfizer/BioNTech), rituximab exposure (OR 0.40, 95%CI 0.16-0.99), and cessation/reduction of anti-rheumatic medication before or after vaccination (OR 4.52, 95%CI 3.28-6.22) were factors independently associated with disease flare (Table 2). In the model using new medication or dosage increase due to flare as dependent variable (Table 2), active disease (LDA, OR 1.49, 95%CI 0.96-2.32; M/HDA, OR 3.13, 95%CI 1.96-5.01; vs remission), not using anti-rheumatic medication (OR 2.73, 95%CI 1.27-5.86), and cessation/reduction of anti-rheumatic medication before or after vaccination (OR 2.20, 95%CI 1.31-3.69) were the only factors independently associated with this more strict flare definition.
Conclusion: Flares of underlying I-RMD following SARS-CoV-2 vaccination were uncommon. Factors associated with potential flares were identified, namely higher disease activity and cessation/reduction of anti-rheumatic medications before or after vaccination. These data will inform SARS-CoV-2 vaccination strategies in patients with I-RMDs.



Disclosures: B. Farisoğulları, None; S. Lawson-Tovey, None; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Carmona, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer; E. Mateus, None; M. Schaefer, None; A. Rodrigues, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; J. Gomez-Puerta, GSK, Galapagos, Pfizer, Janssen, Sanofi, AbbVie, Bristol Myers Squibb, Lilly, Novartis, MSD, Roche; M. Mosca, None; P. Durez, AbbVie, Galapagos, Lilly; L. Trefond, None; T. Goulenok, None; M. Cornalba, None; E. Šteňová, None; I. Bulina, AbbVie/Abbott, AstraZeneca, Janssen, Novartis; E. Strakova, None; J. Zepa, AbbVie/Abbott, Novartis, Janssen, AstraZeneca; N. Roux, None; O. Brocq, None; V. Eric, None; B. Raffeiner, None; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.

