Back
Abstract Session
Session: Abstracts: Muscle Biology, Myositis and Myopathies (2237–2242)
2242: Metabolomics-based Identification of Metabolic Dysfunction in Inclusion Body Myositis
Monday, November 14, 2022
5:45 PM – 5:55 PM Eastern Time
Location: Room 201
.jpg)
Jemima Albayda, MD, RhMSUS
Johns Hopkins University
Baltimore, MD, United States
Presenting Author(s)
Jemima Albayda1, Cissy Zhang1, Pratik Khare1, Brittany Adler1, Erika Darrah1, Lisa Christopher-Stine1, Thomas E Lloyd2 and Anne Le1, 1Johns Hopkins University, Baltimore, MD, 2Johns Hopkins University School of Medicine, Baltimore, MD
Background/Purpose: Sporadic Inclusion Body Myositis (IBM) is the most common myopathy over the age of 50 and is currently refractory to treatment. The pathogenesis of IBM is incompletely understood but is known to share features of both inflammation as well as degeneration. However, the metabolic aspects of IBM are unknown. Hence, there is a need to explore other non-immune aspects contributing to disease advancement for use in disease monitoring and intervention.
Methods: Patients who met the definition of clinico-pathologically defined or clinically defined IBM by ENMC criteria from the Johns Hopkins Myositis Center research registry were included in this study. Controls ( >50 years old) included other myositis and muscular dystrophies confirmed by a rheumatologist or neurologist; healthy donors were obtained from a separate IRB approved study. IBM patients were classified as having mild or early disease if they were independent in ambulation and did not require the use of any implements (n=7); and as advanced disease (n=10) if they required the use of any assistive device. Longitudinal samples from the IBM patients during early and then advanced disease were included when available. Seventeen IBM patients, 5 muscular dystrophies, 5 myositis and 8 healthy controls were included in this study. An untargeted unbiased serum metabolic profiling was carried out to do a global screening for altered pathways in IBM in comparison with non-IBM using LC-MS/MS with reverse-phase chromatography. Metabolite intensities were normalized with protein concentration before comparisons, which were calculated with 2-tailed t test. Significance was defined as p ≤ 0.05.
Results: There was a striking downregulation of serum metabolites involved in glycolysis and oxidative phosphorylation, protein catabolism and nucleotide synthesis in the IBM patients compared to controls. When IBM patients were compared by disease severity, changes were further accentuated in more advanced IBM as compared to milder IBM, as well as longitudinally. Importantly, the severity of the changes was not a function of time but rather subject specific. Specifically, the key compounds of alanine, kynurenine, glycine, 3-phosphohydroxypyruvate, and N-acetylaspartylglutamic acid (NAAG) in the more severe patients. The patient with most deletions in the metabolites was the earliest to become wheelchair dependent.
Conclusion: Our findings point to a striking downregulation of metabolites involved in key energy pathways in IBM when compared with healthy and disease controls. These changes are further accentuated with advancing disease. Further investigation into the mechanisms involved in these altered pathways is needed to understand their possible contribution to disease in IBM.
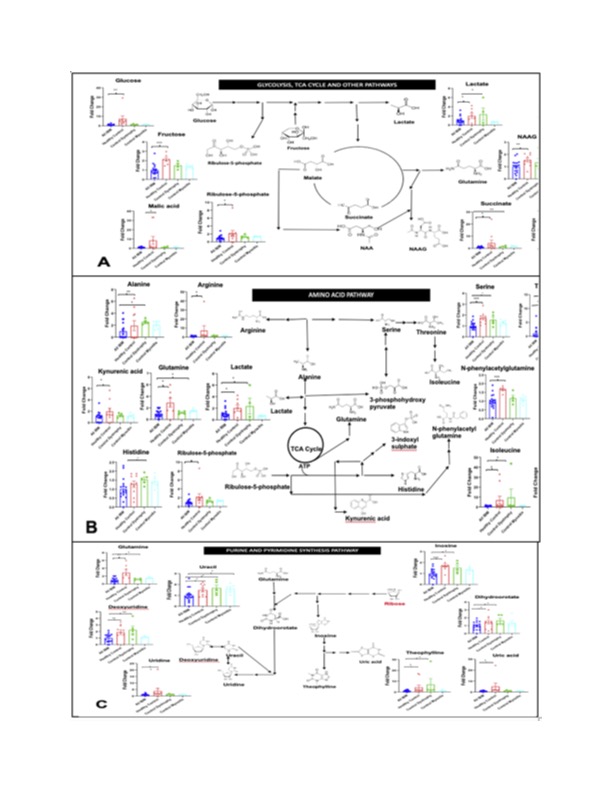 Fig. 1 Downregulation of energy pathways in IBM. Serum metabolomics comparing 17 IBM patients (blue) with age matched 8 healthy (red), 5 dystrophy (green) and 5 myositis (aqua) controls showing the statistically significantly decreased metabolites involved in glycolysis/TCA cycle (A), amino acid pathways (B), and nucleotide synthesis (C). Data are shown as fold change as compared to IBM. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 1 Downregulation of energy pathways in IBM. Serum metabolomics comparing 17 IBM patients (blue) with age matched 8 healthy (red), 5 dystrophy (green) and 5 myositis (aqua) controls showing the statistically significantly decreased metabolites involved in glycolysis/TCA cycle (A), amino acid pathways (B), and nucleotide synthesis (C). Data are shown as fold change as compared to IBM. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
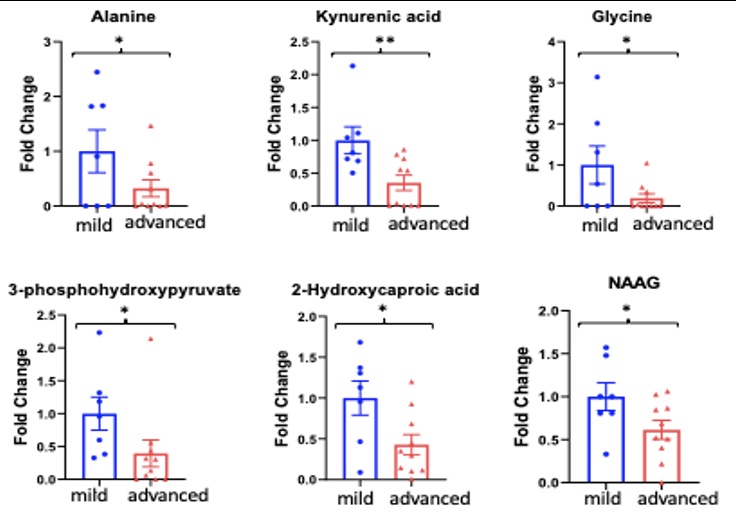 Fig. 2. Serum metabolomics comparing 7 mild IBM (independent with ambulation) with 10 severe IBM (requiring use of assistive devices) patients. Six significantly downregulated metabolites were seen in the advanced group when compared to the mild group. Results shown in fold change.* indicates p < 0.05, ** indicates p < 0.01
Fig. 2. Serum metabolomics comparing 7 mild IBM (independent with ambulation) with 10 severe IBM (requiring use of assistive devices) patients. Six significantly downregulated metabolites were seen in the advanced group when compared to the mild group. Results shown in fold change.* indicates p < 0.05, ** indicates p < 0.01
 Fig. 3. Serum metabolomics comparisons between longitudinal samples for representative metabolites in 4 patients. A- 74 y/o F, 7year interval between samples; B- 70 y/o M, 5-year interval between samples; C- 55 y/o F, 2-year interval between samples; D- 70 y/o M, 7-year interval between samples
Fig. 3. Serum metabolomics comparisons between longitudinal samples for representative metabolites in 4 patients. A- 74 y/o F, 7year interval between samples; B- 70 y/o M, 5-year interval between samples; C- 55 y/o F, 2-year interval between samples; D- 70 y/o M, 7-year interval between samples
Disclosures: J. Albayda, None; C. Zhang, None; P. Khare, None; B. Adler, None; E. Darrah, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; T. Lloyd, None; A. Le, None.
Background/Purpose: Sporadic Inclusion Body Myositis (IBM) is the most common myopathy over the age of 50 and is currently refractory to treatment. The pathogenesis of IBM is incompletely understood but is known to share features of both inflammation as well as degeneration. However, the metabolic aspects of IBM are unknown. Hence, there is a need to explore other non-immune aspects contributing to disease advancement for use in disease monitoring and intervention.
Methods: Patients who met the definition of clinico-pathologically defined or clinically defined IBM by ENMC criteria from the Johns Hopkins Myositis Center research registry were included in this study. Controls ( >50 years old) included other myositis and muscular dystrophies confirmed by a rheumatologist or neurologist; healthy donors were obtained from a separate IRB approved study. IBM patients were classified as having mild or early disease if they were independent in ambulation and did not require the use of any implements (n=7); and as advanced disease (n=10) if they required the use of any assistive device. Longitudinal samples from the IBM patients during early and then advanced disease were included when available. Seventeen IBM patients, 5 muscular dystrophies, 5 myositis and 8 healthy controls were included in this study. An untargeted unbiased serum metabolic profiling was carried out to do a global screening for altered pathways in IBM in comparison with non-IBM using LC-MS/MS with reverse-phase chromatography. Metabolite intensities were normalized with protein concentration before comparisons, which were calculated with 2-tailed t test. Significance was defined as p ≤ 0.05.
Results: There was a striking downregulation of serum metabolites involved in glycolysis and oxidative phosphorylation, protein catabolism and nucleotide synthesis in the IBM patients compared to controls. When IBM patients were compared by disease severity, changes were further accentuated in more advanced IBM as compared to milder IBM, as well as longitudinally. Importantly, the severity of the changes was not a function of time but rather subject specific. Specifically, the key compounds of alanine, kynurenine, glycine, 3-phosphohydroxypyruvate, and N-acetylaspartylglutamic acid (NAAG) in the more severe patients. The patient with most deletions in the metabolites was the earliest to become wheelchair dependent.
Conclusion: Our findings point to a striking downregulation of metabolites involved in key energy pathways in IBM when compared with healthy and disease controls. These changes are further accentuated with advancing disease. Further investigation into the mechanisms involved in these altered pathways is needed to understand their possible contribution to disease in IBM.
 Fig. 1 Downregulation of energy pathways in IBM. Serum metabolomics comparing 17 IBM patients (blue) with age matched 8 healthy (red), 5 dystrophy (green) and 5 myositis (aqua) controls showing the statistically significantly decreased metabolites involved in glycolysis/TCA cycle (A), amino acid pathways (B), and nucleotide synthesis (C). Data are shown as fold change as compared to IBM. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 1 Downregulation of energy pathways in IBM. Serum metabolomics comparing 17 IBM patients (blue) with age matched 8 healthy (red), 5 dystrophy (green) and 5 myositis (aqua) controls showing the statistically significantly decreased metabolites involved in glycolysis/TCA cycle (A), amino acid pathways (B), and nucleotide synthesis (C). Data are shown as fold change as compared to IBM. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. Fig. 2. Serum metabolomics comparing 7 mild IBM (independent with ambulation) with 10 severe IBM (requiring use of assistive devices) patients. Six significantly downregulated metabolites were seen in the advanced group when compared to the mild group. Results shown in fold change.* indicates p < 0.05, ** indicates p < 0.01
Fig. 2. Serum metabolomics comparing 7 mild IBM (independent with ambulation) with 10 severe IBM (requiring use of assistive devices) patients. Six significantly downregulated metabolites were seen in the advanced group when compared to the mild group. Results shown in fold change.* indicates p < 0.05, ** indicates p < 0.01 Fig. 3. Serum metabolomics comparisons between longitudinal samples for representative metabolites in 4 patients. A- 74 y/o F, 7year interval between samples; B- 70 y/o M, 5-year interval between samples; C- 55 y/o F, 2-year interval between samples; D- 70 y/o M, 7-year interval between samples
Fig. 3. Serum metabolomics comparisons between longitudinal samples for representative metabolites in 4 patients. A- 74 y/o F, 7year interval between samples; B- 70 y/o M, 5-year interval between samples; C- 55 y/o F, 2-year interval between samples; D- 70 y/o M, 7-year interval between samplesDisclosures: J. Albayda, None; C. Zhang, None; P. Khare, None; B. Adler, None; E. Darrah, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; T. Lloyd, None; A. Le, None.

