Back
Abstract Session
Session: Abstracts: Patient Outcomes, Preferences, and Attitudes: Patient Priorities and Preferences: Interventions and Transformation (2243–2247)
2247: Priority Research Topics for Vaccine Uptake Among Adults with Autoimmune Conditions
Monday, November 14, 2022
5:30 PM – 5:40 PM Eastern Time
Location: Room 114 Nutter Theatre
.png)
William Benjamin Nowell, PhD, MSW
Global Healthy Living Foundation
Nyack, NY, United States
Presenting Author(s)
Shilpa Venkatachalam1, William Benjamin Nowell2, Shubhasree Banerjee3, Kelly Gavigan4, Laura Stradford2, Jennifer Gordon5, Lisa Emerich6, Hope Sullivan7, Ashira Blazer8, Brittany Banbury9, Vandana Dronadula1, Kimberly Weaver10, Angela Degrassi4, Peter Merkel3, Robert McBurney11, Mike Kappelman10, Jeffrey Curtis12 and Michael George3, 1Global Healthy Living Foundation, New York, NY, 2Global Healthy Living Foundation, Nyack, NY, 3University of Pennsylvania, Philadelphia, PA, 4Global Healthy Living Foundation, Upper Nyack, NY, 5Vasculitis Foundation, Philadelphia, 6iConquerMS, Waltham, MA, 7Inflammatory Bowel Disease (IBD) Partners, Chapel Hill, NC, 8Hospital for Special Surgery, New York, NY, 9NYU Langone, Fort Lee, NJ, 10School of Medicine, University of North Carolina (UNC), Chapel Hill, NC, 11Accelerated Cure Project for Multiple Sclerosis, Waltham, MA, 12University of Alabama at Birmingham, Hoover, AL
Background/Purpose: Compared to the general population, adults living with autoimmune disease are at nearly twice the risk of vaccine-preventable infections, making this a high priority vaccination group. The objective of this study was to prioritize topics for future patient-centered research to reduce vaccine hesitancy and increase uptake of vaccines for conditions such as pneumococcal pneumonia, influenza, zoster, human papillomavirus, and SARS-CoV-2 among adults living with autoimmune conditions, as informed by a range of stakeholders in the autoimmune health community.
Methods: We convened a steering committee (SC) of clinicians and patients with representation from rheumatic diseases (PsA, RA, vasculitis), inflammatory bowel disease, and multiple sclerosis. Through literature review and iterative discussions, SC members identified 33 vaccine uptake/hesitancy research topics in four domains. A larger multistakeholder group including patients and patient advocates (Patients/Advocates); clinicians and researchers (Clinicians/Researchers); policy makers, regulators, and vaccine manufacturers (Other Stakeholders) was convened to conduct a modified online Delphi process with two rounds currently completed. In each round, group members rated each topic on a 9-point scale (1-3 = not important, 4-6 = important but not critical, 7-9 = critical) and could propose additional topics for inclusion in the next round. When completing round 2 (R2), participants were able to view their ratings from the previous round and view aggregate ratings from their respective stakeholder group before confirming or changing how they rated each topic. Frequency analysis and comparisons across the three stakeholder groups (Patients/Clinicians/Other) was conducted.
Results: A total of 34 stakeholders participated in both R1 and R2 of the Delphi to rate the 33 R1 and 34 R2 topics. Overall, six topics, representing at least one topic from each domain, were rated as critical by > 90% of stakeholders, including topics related to vaccine effectiveness in autoimmune disease, beliefs about safety, myths and misinformation, trust in the healthcare system, barriers to access, and vaccine safety profiles (Table 1). The Patient/Patient Advocate group unanimously rated three topics as critical: How myths or misinformation about vaccines affect vaccine uptake, How source of information about vaccines affects vaccine uptake, and How perceived transparency of information about vaccines affect vaccine uptake. The Researchers/Clinicians group unanimously rated How well a vaccine works for adults with autoimmune conditions as critical.
Conclusion: Topics identified as critical across stakeholder groups can inform future research efforts to decrease vaccine hesitancy and improve uptake of relevant vaccines for adults with autoimmune conditions, a high-priority group for vaccinations.
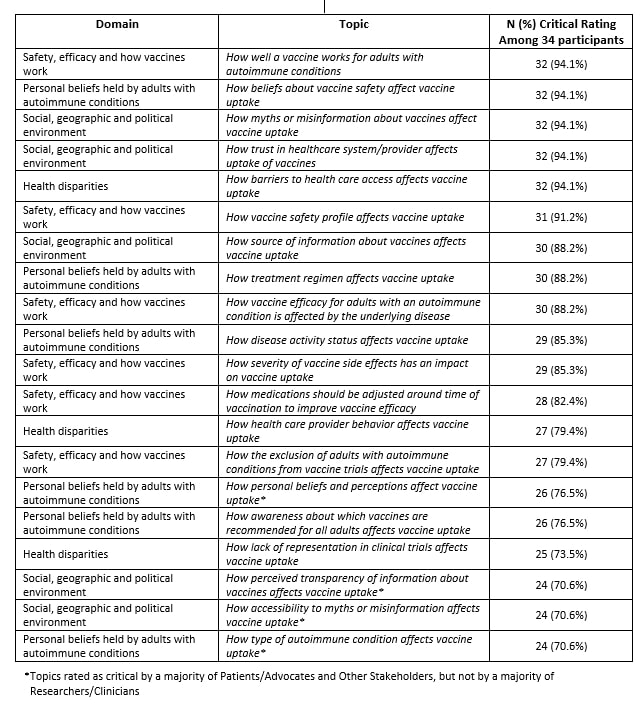 Table 1. Research Topics Rated as Critical for Vaccine Uptake Among Adults with Autoimmune Conditions
Table 1. Research Topics Rated as Critical for Vaccine Uptake Among Adults with Autoimmune Conditions
Disclosures: S. Venkatachalam, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; S. Banerjee, None; K. Gavigan, None; L. Stradford, Global Healthy Living Foundation; J. Gordon, None; L. Emerich, None; H. Sullivan, Merck/MSD; A. Blazer, None; B. Banbury, None; V. Dronadula, None; K. Weaver, None; A. Degrassi, None; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate; R. McBurney, None; M. Kappelman, None; J. Curtis, AbbVie/Abbott, Amgen, ArthritisPower, Aqtual, Bendcare, Bristol-Myers Squibb(BMS), CorEvitas, FASTER, GlaxoSmithKlein(GSK), IlluminationHealth, Janssen, Labcorp, Eli Lilly, Myriad, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB, United Rheumatology; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx.
Background/Purpose: Compared to the general population, adults living with autoimmune disease are at nearly twice the risk of vaccine-preventable infections, making this a high priority vaccination group. The objective of this study was to prioritize topics for future patient-centered research to reduce vaccine hesitancy and increase uptake of vaccines for conditions such as pneumococcal pneumonia, influenza, zoster, human papillomavirus, and SARS-CoV-2 among adults living with autoimmune conditions, as informed by a range of stakeholders in the autoimmune health community.
Methods: We convened a steering committee (SC) of clinicians and patients with representation from rheumatic diseases (PsA, RA, vasculitis), inflammatory bowel disease, and multiple sclerosis. Through literature review and iterative discussions, SC members identified 33 vaccine uptake/hesitancy research topics in four domains. A larger multistakeholder group including patients and patient advocates (Patients/Advocates); clinicians and researchers (Clinicians/Researchers); policy makers, regulators, and vaccine manufacturers (Other Stakeholders) was convened to conduct a modified online Delphi process with two rounds currently completed. In each round, group members rated each topic on a 9-point scale (1-3 = not important, 4-6 = important but not critical, 7-9 = critical) and could propose additional topics for inclusion in the next round. When completing round 2 (R2), participants were able to view their ratings from the previous round and view aggregate ratings from their respective stakeholder group before confirming or changing how they rated each topic. Frequency analysis and comparisons across the three stakeholder groups (Patients/Clinicians/Other) was conducted.
Results: A total of 34 stakeholders participated in both R1 and R2 of the Delphi to rate the 33 R1 and 34 R2 topics. Overall, six topics, representing at least one topic from each domain, were rated as critical by > 90% of stakeholders, including topics related to vaccine effectiveness in autoimmune disease, beliefs about safety, myths and misinformation, trust in the healthcare system, barriers to access, and vaccine safety profiles (Table 1). The Patient/Patient Advocate group unanimously rated three topics as critical: How myths or misinformation about vaccines affect vaccine uptake, How source of information about vaccines affects vaccine uptake, and How perceived transparency of information about vaccines affect vaccine uptake. The Researchers/Clinicians group unanimously rated How well a vaccine works for adults with autoimmune conditions as critical.
Conclusion: Topics identified as critical across stakeholder groups can inform future research efforts to decrease vaccine hesitancy and improve uptake of relevant vaccines for adults with autoimmune conditions, a high-priority group for vaccinations.
 Table 1. Research Topics Rated as Critical for Vaccine Uptake Among Adults with Autoimmune Conditions
Table 1. Research Topics Rated as Critical for Vaccine Uptake Among Adults with Autoimmune ConditionsDisclosures: S. Venkatachalam, None; W. Nowell, Global Healthy Living Foundation, AbbVie Inc., Amgen, Eli Lilly; S. Banerjee, None; K. Gavigan, None; L. Stradford, Global Healthy Living Foundation; J. Gordon, None; L. Emerich, None; H. Sullivan, Merck/MSD; A. Blazer, None; B. Banbury, None; V. Dronadula, None; K. Weaver, None; A. Degrassi, None; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate; R. McBurney, None; M. Kappelman, None; J. Curtis, AbbVie/Abbott, Amgen, ArthritisPower, Aqtual, Bendcare, Bristol-Myers Squibb(BMS), CorEvitas, FASTER, GlaxoSmithKlein(GSK), IlluminationHealth, Janssen, Labcorp, Eli Lilly, Myriad, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB, United Rheumatology; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx.

