Back
Abstract Session
Session: Abstracts: Infection-related Rheumatic Disease (2272–2275)
2275: Three Dose SARS-CoV-2 Vaccination Is Associated with Reduced Risk of Breakthrough COVID-19 During Omicron Wave in Patients with Autoimmune Diseases
Monday, November 14, 2022
5:45 PM – 5:55 PM Eastern Time
Location: Exhibit Hall A
- CC
Caoilfhionn M. Connolly, MD, MSc
Johns Hopkins University School of Medicine
Baltimore, MD, United States
Presenting Author(s)
Caoilfhionn Connolly1, Rachel Wallwork1, Teresa Po-Yu Chiang1, Mayan Teles1, Jennifer Alejo1, Allan Massie2, Ami Shah3, Jemima Albayda1, Lisa Christopher-Stine1, Dorry Segev2, William Werbel1 and Julie Paik1, 1Johns Hopkins University, Baltimore, MD, 2NYU Langone Health, New York, NY, 3Johns Hopkins Rheumatology, Baltimore, MD
Background/Purpose: While SARS-CoV-2 vaccination mitigates the risk of severe COVID-19, vaccinated patients with autoimmune diseases are more susceptible to infection despite vaccination ("breakthrough" COVID-19) and endure poorer outcomes. With the emergence of the highly transmissible SARS-CoV-2 variants of concern (VOC), rates of breakthrough COVID-19 have increased. The primary purpose of this study was to assess the incidence of, and clinical factors associated with, breakthrough COVID-19 in persons with autoimmune disease, with focus on the Omicron variant wave (December 2021 – March 2022). Secondarily we sought to characterize the association of preceding anti-SARS-CoV-2 antibody titers on the incidence of breakthrough infection.
Methods: Adult patients with autoimmune diseases who received at least two SARS-CoV-2 mRNA vaccine doses (D2) were followed from 1/28/2021-03/17/2022. Serial post-vaccination antibody sampling was undertaken using a semi-quantitative anti-spike assay (anti-RBD, range< 0.8-2500 units/mL). Participants were surveyed periodically for breakthrough COVID-19 (infection occurring at least two weeks after two-dose of BNT162b2/mRNA-1273 vaccine). Omicron variant wave was defined as 12/01/2021-03/17/2022. Participants who underwent antibody testing in the four weeks prior to 12/1/2021 were included in a sub-analysis, with the closest anti-RBD measurement prior to 12/1/2021 was defined as pre-Omicron titer. Associations between breakthrough and participant characteristics were compared using Wilcoxon rank-sum test and Fisher's exact test as appropriate. We modeled the time to infection beginning on 12/1/2021 using Cox proportional hazards model.
Results: We studied 1273 participants (median age 49.4 [IQR 39.3,59.7]; 90% female). 218(17.1%) reported breakthrough; 172 (78.9% of infections) occurred after 12/01/2022 (Table 1). Most symptoms were graded as mild (40.3%) or moderate (55.4%); there were six reports of severe symptoms (3.5%) requiring hospitalization without ICU admission. Participants experiencing breakthrough, were more often younger(p< 0.001), Hispanic ethnicity(p=0.03), and had not yet received a third vaccine dose (D3) (p< 0.001). Receipt of D3 (aHR 0.58; 95% CI 0.41-0.76; p=0.001) (Figure 1) and older age (per decade increase) (aHR 0.71, 95% CI 0.64-0.80; p< 0.001) were associated with reduced risk of breakthrough during Omicron wave (Table 2). Among the 470 participants who underwent anti-RBD testing in the four weeks prior to 12/01/ 2021, 20 (4.3%) were seronegative, while 386 (82.1%), 356 (75.7%) and 305 (64.9%) had antibody titers of ≥500, ≥1000 and ≥2500 U/mL, respectively. Among those with anti-RBD≥2500U/mL, a smaller proportion reported COVID-19 compared to those with anti-RBD< 2500U/mL (7% versus 13.9%; p=0.02)
Conclusion: Breakthrough COVID-19 was reported by 17.1%, predominately occurring during the Omicron wave. D3 was associated with a significantly reduced risk of infection, as was preceding higher-level anti-RBD response. These findings support the importance of additional and booster vaccination to reduce SARS-CoV-2 infection.
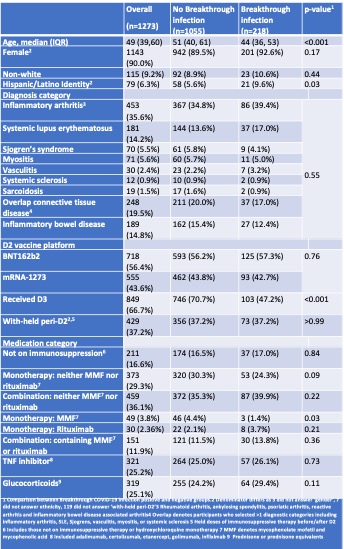 Table 1. Characteristics of 1273 RMD patients, stratified by presence/absence of COVID-19 breakthrough
Table 1. Characteristics of 1273 RMD patients, stratified by presence/absence of COVID-19 breakthrough
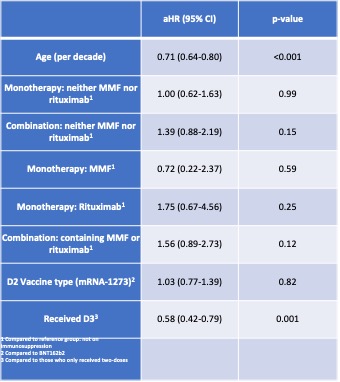 Table 2. Hazard ratio with 95% confidence interval of multivariable Cox proportional hazards regression analysis.
Table 2. Hazard ratio with 95% confidence interval of multivariable Cox proportional hazards regression analysis.
.jpg) Figure 1. Infection free survival Kaplan Meier curves comparing breakthrough COVID-19 since 12/01/2021 among participants who received two-dose versus three-dose vaccination
Figure 1. Infection free survival Kaplan Meier curves comparing breakthrough COVID-19 since 12/01/2021 among participants who received two-dose versus three-dose vaccination
Disclosures: C. Connolly, None; R. Wallwork, None; T. Po-Yu Chiang, None; M. Teles, None; J. Alejo, None; A. Massie, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; J. Albayda, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; D. Segev, Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, Caredx, Transmedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda, Bridge to Life; W. Werbel, None; J. Paik, Pfizer Inc, Kezar Inc, Roivant, Argenx, Alexion Inc.
Background/Purpose: While SARS-CoV-2 vaccination mitigates the risk of severe COVID-19, vaccinated patients with autoimmune diseases are more susceptible to infection despite vaccination ("breakthrough" COVID-19) and endure poorer outcomes. With the emergence of the highly transmissible SARS-CoV-2 variants of concern (VOC), rates of breakthrough COVID-19 have increased. The primary purpose of this study was to assess the incidence of, and clinical factors associated with, breakthrough COVID-19 in persons with autoimmune disease, with focus on the Omicron variant wave (December 2021 – March 2022). Secondarily we sought to characterize the association of preceding anti-SARS-CoV-2 antibody titers on the incidence of breakthrough infection.
Methods: Adult patients with autoimmune diseases who received at least two SARS-CoV-2 mRNA vaccine doses (D2) were followed from 1/28/2021-03/17/2022. Serial post-vaccination antibody sampling was undertaken using a semi-quantitative anti-spike assay (anti-RBD, range< 0.8-2500 units/mL). Participants were surveyed periodically for breakthrough COVID-19 (infection occurring at least two weeks after two-dose of BNT162b2/mRNA-1273 vaccine). Omicron variant wave was defined as 12/01/2021-03/17/2022. Participants who underwent antibody testing in the four weeks prior to 12/1/2021 were included in a sub-analysis, with the closest anti-RBD measurement prior to 12/1/2021 was defined as pre-Omicron titer. Associations between breakthrough and participant characteristics were compared using Wilcoxon rank-sum test and Fisher's exact test as appropriate. We modeled the time to infection beginning on 12/1/2021 using Cox proportional hazards model.
Results: We studied 1273 participants (median age 49.4 [IQR 39.3,59.7]; 90% female). 218(17.1%) reported breakthrough; 172 (78.9% of infections) occurred after 12/01/2022 (Table 1). Most symptoms were graded as mild (40.3%) or moderate (55.4%); there were six reports of severe symptoms (3.5%) requiring hospitalization without ICU admission. Participants experiencing breakthrough, were more often younger(p< 0.001), Hispanic ethnicity(p=0.03), and had not yet received a third vaccine dose (D3) (p< 0.001). Receipt of D3 (aHR 0.58; 95% CI 0.41-0.76; p=0.001) (Figure 1) and older age (per decade increase) (aHR 0.71, 95% CI 0.64-0.80; p< 0.001) were associated with reduced risk of breakthrough during Omicron wave (Table 2). Among the 470 participants who underwent anti-RBD testing in the four weeks prior to 12/01/ 2021, 20 (4.3%) were seronegative, while 386 (82.1%), 356 (75.7%) and 305 (64.9%) had antibody titers of ≥500, ≥1000 and ≥2500 U/mL, respectively. Among those with anti-RBD≥2500U/mL, a smaller proportion reported COVID-19 compared to those with anti-RBD< 2500U/mL (7% versus 13.9%; p=0.02)
Conclusion: Breakthrough COVID-19 was reported by 17.1%, predominately occurring during the Omicron wave. D3 was associated with a significantly reduced risk of infection, as was preceding higher-level anti-RBD response. These findings support the importance of additional and booster vaccination to reduce SARS-CoV-2 infection.
 Table 1. Characteristics of 1273 RMD patients, stratified by presence/absence of COVID-19 breakthrough
Table 1. Characteristics of 1273 RMD patients, stratified by presence/absence of COVID-19 breakthrough Table 2. Hazard ratio with 95% confidence interval of multivariable Cox proportional hazards regression analysis.
Table 2. Hazard ratio with 95% confidence interval of multivariable Cox proportional hazards regression analysis..jpg) Figure 1. Infection free survival Kaplan Meier curves comparing breakthrough COVID-19 since 12/01/2021 among participants who received two-dose versus three-dose vaccination
Figure 1. Infection free survival Kaplan Meier curves comparing breakthrough COVID-19 since 12/01/2021 among participants who received two-dose versus three-dose vaccination Disclosures: C. Connolly, None; R. Wallwork, None; T. Po-Yu Chiang, None; M. Teles, None; J. Alejo, None; A. Massie, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; J. Albayda, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; D. Segev, Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, Caredx, Transmedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda, Bridge to Life; W. Werbel, None; J. Paik, Pfizer Inc, Kezar Inc, Roivant, Argenx, Alexion Inc.

