Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1020: Screening Tools for Spondyloarthritis in Patients with Psoriasis, IBD, and Uveitis - A Scoping Review
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- VK
Vartika Kesarwani, MBBS
University of Connecticut
Hartford, CT, United States
Abstract Poster Presenter(s)
Vartika Kesarwani1, Stephanie Sinnappan2, M. Elaine Husni3, Michael Weisman4 and Joerg Ermann5, 1University of Connecticut, Farmington, CT, 2Brigham and Women's Hospital, Boston, MA, 3Cleveland Clinic, Cleveland, OH, 4Adjunct Professor of Medicine, Stanford University; Distinguished Professor of Medicine Emeritus, David Geffen School of Medicine at UCLA, Los Angeles, CA, 5Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Background/Purpose: Extra-articular manifestations of spondyloarthritis (SpA) may precede the development of articular features. Patients seen in dermatology, gastroenterology, and ophthalmology clinics for psoriasis, IBD, or uveitis may have undiagnosed SpA. The goal of this review was to assess the available evidence and compare the design of existing screening tools for SpA in patients with psoriasis, IBD, and uveitis. We further sought to identify relevant factors that may influence the performance of these instruments and aid in developing more robust SpA screening tools in the future.
Methods: We followed the PRISMA guideline extension for scoping reviews. We searched PubMed, EMBASE, and Web of Science from inception to January 2022. Two independent reviewers identified and screened the studies for eligibility. Data regarding the study type, development method, characteristics of the screening tool, and the performance parameters were extracted.
Results: Fifty-two studies were included. Eighteen were descriptions of new screening tools, 23 were validation studies, 8 were comparative analyses of previously described tools, and 3 were systematic reviews. We identified 13 screening tools for PsA, 3 tools for SpA screening in IBD, and 2 tools for SpA screening in uveitis (Table 1). Most tools were developed using expert opinion and literature review. PEST, pAsQ, CONTEST, SiPAT, SiPAS, and STRIPP were formulated by combining previously described tools. Only PASE and IBIS-Q used patient input in the development phase.
Almost all screening tools are questionnaires to be completed by the patients. A single tool, DUET, is an algorithm applied by a physician. The median number of items included was 6 (range 3 – 16). Most tools use simple yes/no questions. The PASE uses a five-point Likert scale, and PsA-Disk uses an 11-point numerical scale. The screening tools showed variability in identifying different features of SpA (Table 2). Three of the 18 tools used visual aids such as mannequins or photographs (Table 3). The completion time was reported for 5 of the 18 tools with an average of < 5 minutes. The sensitivity and specificity of all the screening tools were largely equivalent in the development cohorts ranging from 83 to 97% and 71 to 97%, respectively. The sensitivity and specificity were generally lower in the subsequent validation studies.
Conclusion: There is no single SpA screening tool that can be applied to all patients with any extra-articular manifestation. Most screening tools were for PsA with a paucity of SpA screening tools for patients with IBD or uveitis. Not all tools screened for axial involvement. We conclude that an ideal screening tool should include questions related to axial involvement, peripheral arthritis, enthesitis, and dactylitis. It might be possible to improve performance by eliminating questions not specific to inflammatory arthritis, including most discriminatory questions, and utilizing visual aids to improve agreement between doctors and patients. Patient-focused screening tools need to be easy to understand and quickly completed by the patient during the clinic visit before provider engagement.
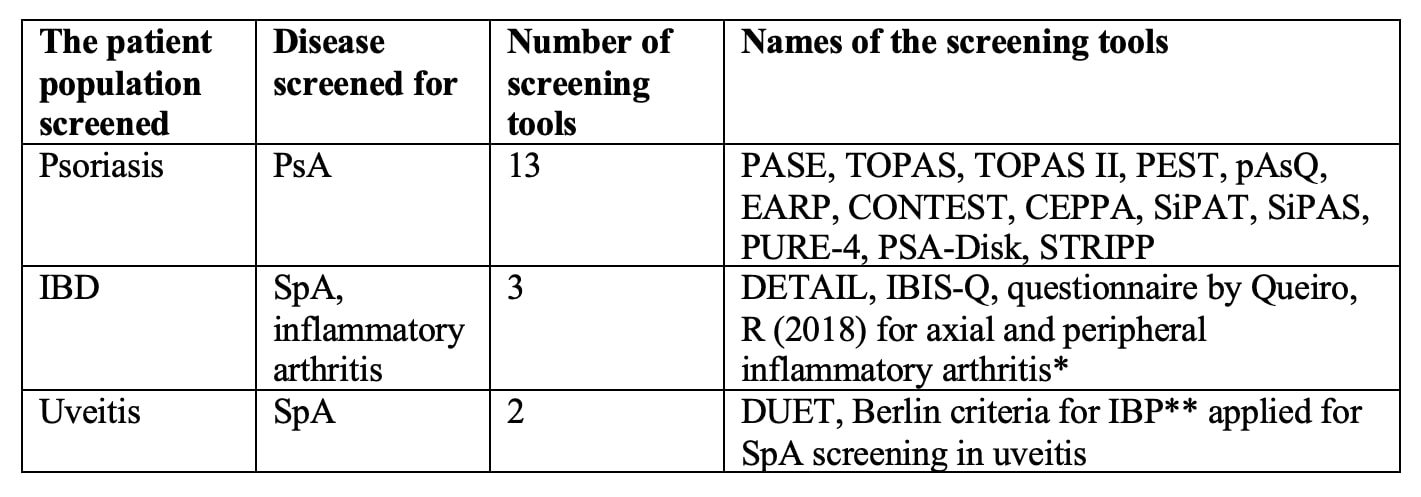 Table 1: SpA screening tools in patients with psoriasis, IBD, and uveitis. *2 questionnaires consisted of 3 questions each for axial and peripheral inflammatory arthritis: **Inflammatory back pain.
Table 1: SpA screening tools in patients with psoriasis, IBD, and uveitis. *2 questionnaires consisted of 3 questions each for axial and peripheral inflammatory arthritis: **Inflammatory back pain.
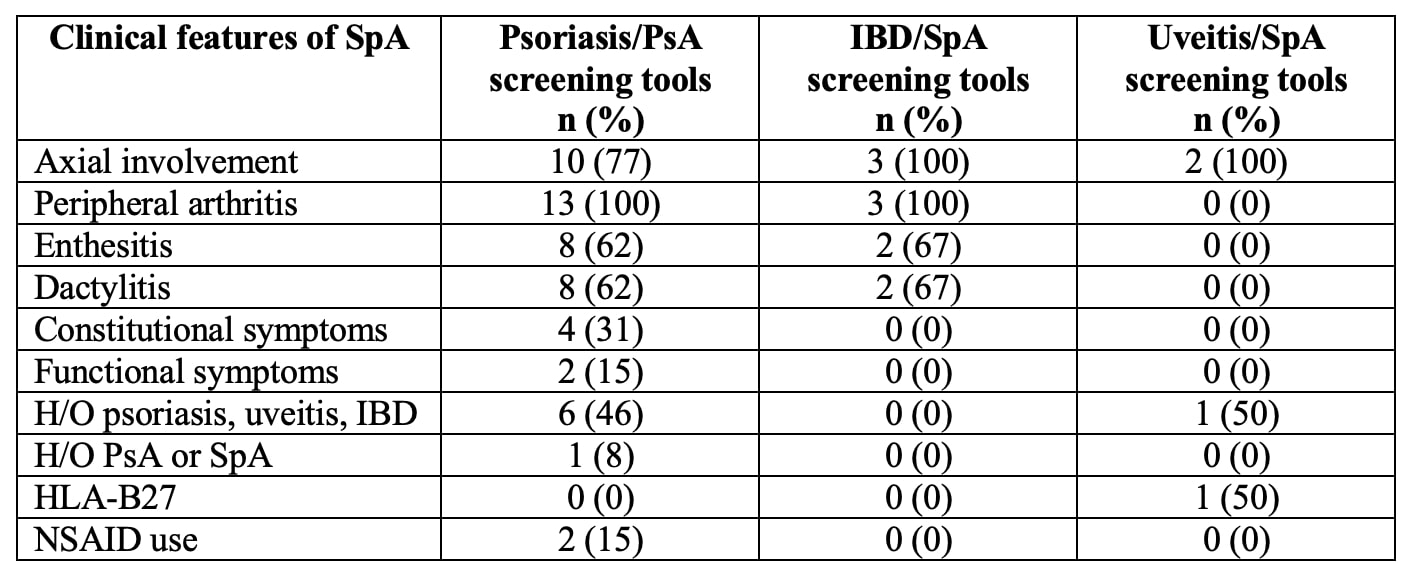 Table 2: Analysis of questions included in the screening tools.
Table 2: Analysis of questions included in the screening tools.
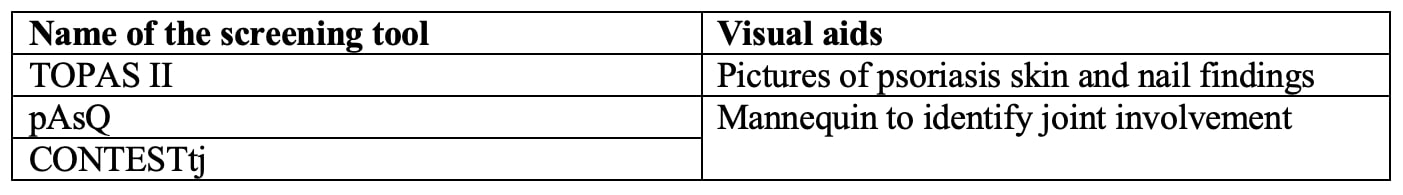 Table 3: Visual aids used in the screening tools.
Table 3: Visual aids used in the screening tools.
Disclosures: V. Kesarwani, None; S. Sinnappan, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; M. Weisman, None; J. Ermann, Novartis, Pfizer, AbbVie/Abbott, UCB, Eli Lilly.
Background/Purpose: Extra-articular manifestations of spondyloarthritis (SpA) may precede the development of articular features. Patients seen in dermatology, gastroenterology, and ophthalmology clinics for psoriasis, IBD, or uveitis may have undiagnosed SpA. The goal of this review was to assess the available evidence and compare the design of existing screening tools for SpA in patients with psoriasis, IBD, and uveitis. We further sought to identify relevant factors that may influence the performance of these instruments and aid in developing more robust SpA screening tools in the future.
Methods: We followed the PRISMA guideline extension for scoping reviews. We searched PubMed, EMBASE, and Web of Science from inception to January 2022. Two independent reviewers identified and screened the studies for eligibility. Data regarding the study type, development method, characteristics of the screening tool, and the performance parameters were extracted.
Results: Fifty-two studies were included. Eighteen were descriptions of new screening tools, 23 were validation studies, 8 were comparative analyses of previously described tools, and 3 were systematic reviews. We identified 13 screening tools for PsA, 3 tools for SpA screening in IBD, and 2 tools for SpA screening in uveitis (Table 1). Most tools were developed using expert opinion and literature review. PEST, pAsQ, CONTEST, SiPAT, SiPAS, and STRIPP were formulated by combining previously described tools. Only PASE and IBIS-Q used patient input in the development phase.
Almost all screening tools are questionnaires to be completed by the patients. A single tool, DUET, is an algorithm applied by a physician. The median number of items included was 6 (range 3 – 16). Most tools use simple yes/no questions. The PASE uses a five-point Likert scale, and PsA-Disk uses an 11-point numerical scale. The screening tools showed variability in identifying different features of SpA (Table 2). Three of the 18 tools used visual aids such as mannequins or photographs (Table 3). The completion time was reported for 5 of the 18 tools with an average of < 5 minutes. The sensitivity and specificity of all the screening tools were largely equivalent in the development cohorts ranging from 83 to 97% and 71 to 97%, respectively. The sensitivity and specificity were generally lower in the subsequent validation studies.
Conclusion: There is no single SpA screening tool that can be applied to all patients with any extra-articular manifestation. Most screening tools were for PsA with a paucity of SpA screening tools for patients with IBD or uveitis. Not all tools screened for axial involvement. We conclude that an ideal screening tool should include questions related to axial involvement, peripheral arthritis, enthesitis, and dactylitis. It might be possible to improve performance by eliminating questions not specific to inflammatory arthritis, including most discriminatory questions, and utilizing visual aids to improve agreement between doctors and patients. Patient-focused screening tools need to be easy to understand and quickly completed by the patient during the clinic visit before provider engagement.
 Table 1: SpA screening tools in patients with psoriasis, IBD, and uveitis. *2 questionnaires consisted of 3 questions each for axial and peripheral inflammatory arthritis: **Inflammatory back pain.
Table 1: SpA screening tools in patients with psoriasis, IBD, and uveitis. *2 questionnaires consisted of 3 questions each for axial and peripheral inflammatory arthritis: **Inflammatory back pain.  Table 2: Analysis of questions included in the screening tools.
Table 2: Analysis of questions included in the screening tools.  Table 3: Visual aids used in the screening tools.
Table 3: Visual aids used in the screening tools. Disclosures: V. Kesarwani, None; S. Sinnappan, None; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; M. Weisman, None; J. Ermann, Novartis, Pfizer, AbbVie/Abbott, UCB, Eli Lilly.

