Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0705: Severity of SARS-CoV-2 Omicron Breakthrough Infections in Patients with Rheumatic Immune-mediated Inflammatory Diseases and Healthy Controls: Data from a Prospective Cohort Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- LB
Laura Boekel, BSc
Vrije Universiteit Amsterdam
Amsterdam, Netherlands
Abstract Poster Presenter(s)
Laura Boekel1, Yaëlle Besten1, Femke Hooijberg1, Rosa Wartena1, Maurice Steenhuis2, Erik Vogelzang3, Maureen Leeuw1, Sadaf Atiqi1, Sander Tas4, Willem Lems5, Marieke van Ham2, Filip Eftimov3, Eileen Stalman3, Luuk Wieske3, Taco Kuijpers3, Alexandre Voskuyl6, Ronald van Vollenhoven7, Martijn Gerritsen1, Charlotte Krieckaert1, Theo Rispens2, Maarten Boers8, Michael Nurmohamed9 and Gertjan Wolbink1, 1Amsterdam Rheumatology and immunology Center, location Reade, Amsterdam, Netherlands, 2Sanquin Research, Amsterdam, Netherlands, 3Amsterdam UMC, location AMC, Amsterdam, Netherlands, 4Amsterdam Rheumatology and immunology Center, location AMC, Amsterdam, Netherlands, 5Amsterdam Rheumatology and immunology Center, location VUMC, Amsterdam, Netherlands, 6Amsterdam UMC, Amsterdam, Netherlands, 7Amsterdam University Medical Centers, Amsterdam, Netherlands, 8Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 9Amsterdam University Medical Center, Kortenhoef, Netherlands
Background/Purpose: The Omicron (B.1.1.529) variant of SARS-CoV-2 is associated with substantially lower hospitalization rates compared with previous variants of SARS-CoV-2 (i.e., Wildtype, Alpha [B.1.1.7] and Delta [B.1.617.2] variant) in people of the general population, but data in patients with rheumatic immune-mediated inflammatory diseases (IMIDs) are still scarce. Therefore, we compared the severity of Omicron breakthrough infections between patients with rheumatic IMIDs and healthy controls using data from a large ongoing prospective cohort study.
Methods: In April 2020, all adult patients with rheumatic IMIDs from the Amsterdam Rheumatology and Immunology Center (ARC) in the Netherlands were invited to participate. Patients were asked to recruit their own control participant of the same sex and comparable age. Data on SARS-CoV-2 infections were collected between April 2020 and April 2022 using digital questionnaires. Serum samples were collected during the entire follow-up period and analyzed for the presence of SARS-CoV-2 specific antibodies. Vaccination-naïve SARS-CoV-2 infections were defined as PCR- or serological confirmed infections before SARS-CoV-2 vaccination. As all data on vaccination-naïve infections were collected before the emergence of the Delta variant in the Netherlands, these infections were considered Wildtype or Alpha variant infections. Breakthrough SARS-CoV-2 infections were defined as PCR- or antigen confirmed infections detected at least 14 days after SARS-CoV-2 vaccination. Breakthrough infections were classified as Delta or Omicron infections based on publicly available data on the predominant circulating SARS-CoV-2 variant in the Netherlands. Logistic regression and Fisher's exact test were used for statistical analyses.
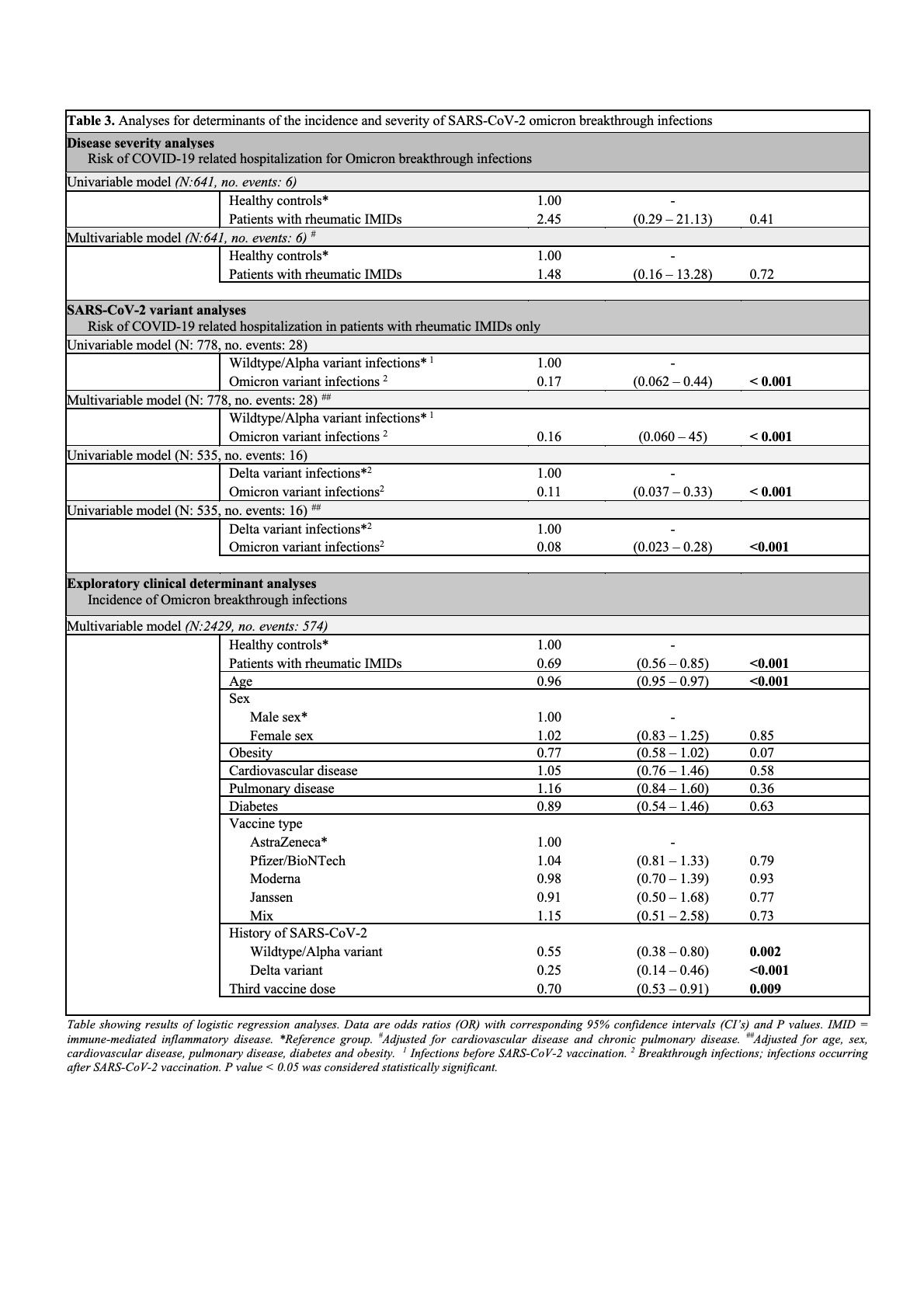
Results: In total, 1882 consecutive patients with rheumatic IMIDs and 706 healthy controls were included for analyses on Omicron breakthrough infections. Omicron breakthrough infections were detected in 431 (23%) of 1882 IMID patients and 210 (30%) of 706 healthy controls. Hospitalization was required in 5 (1.2%) of 431 patients and in 1 (0.5%) of 210 controls (aOR: 1.5, 95% CI: 0.2 – 13, P 0.7; Table 1). Anti-CD20 therapy was significantly associated with a higher risk of hospitalization or death (P < 0.001). Hospitalization rates of Omicron breakthrough infections in patients with rheumatic IMIDs were lower compared to Wildtype/Alpha vaccination-naïve infections (aOR: 0.2, 95% CI: 0.06 – 0.5, P < 0.001) and Delta breakthrough infections (aOR: 0.08, 95% CI: 0.02 – 0.3, P < 0.001) (Figure 1 and Table 1). Lastly, a history of Wildtype/Alpha vaccination naïve infections, Delta breakthrough infections and third SARS-CoV-2 vaccine doses were associated with a lower risk of Omicron breakthrough infections (Table 1).
Conclusion: We showed that the risk of a severe disease course of Omicron breakthrough infections is substantially lower compared with previous SARS-CoV-2 variants, and that the risk of severe disease is comparable for most patients with rheumatic IMIDs and healthy controls. However, patients receiving anti-CD20 therapy remain at increased risk of severe COVID-19, even when infected with the Omicron variant of SARS-CoV-2.
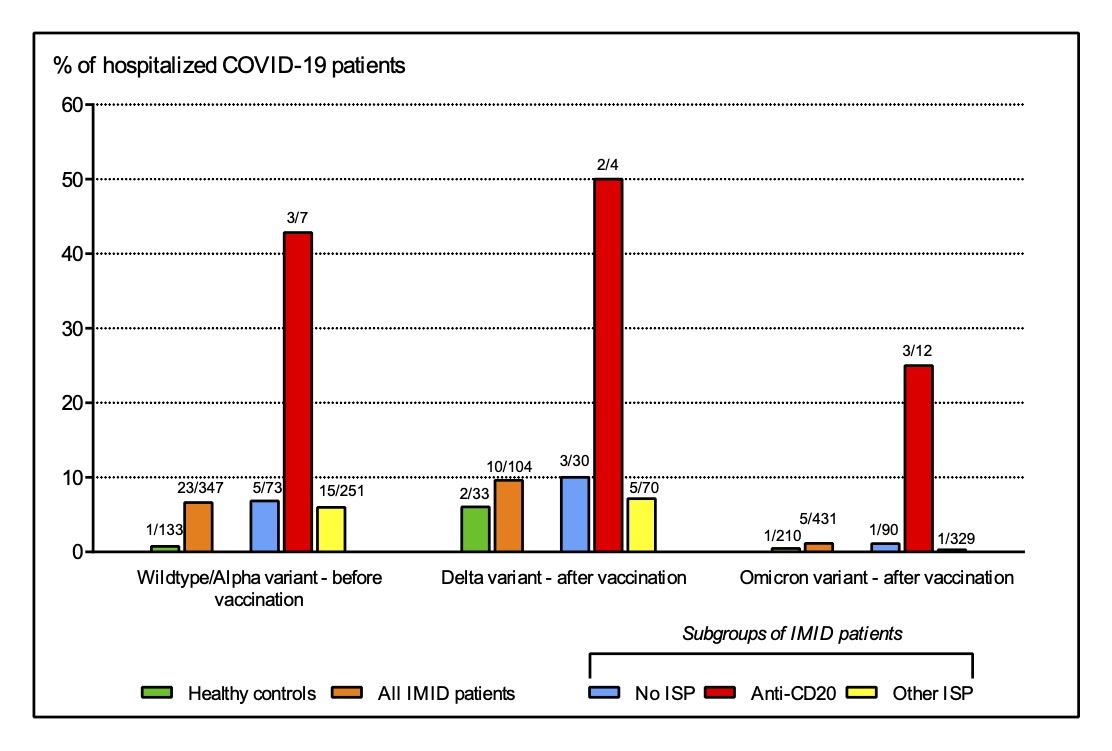 Figure 3. Hospitalization rates of SARS-CoV-2 infections.
Figure 3. Hospitalization rates of SARS-CoV-2 infections.
Figure showing the proportion of patients with rheumatic IMIDs (orange) and healthy controls (green) who required hospitalization after infection with SARS-CoV-2 Wildtype/Alpha variant before vaccination (left panel), SARS-CoV-2 Delta variant after vaccination (middle panel), and SARS-CoV-2 Omicron variant after vaccination (right panel). Patients were further divided into subgroups based on immunosuppressive treatment: no immunosuppressive treatment (blue), treatment with anti-CD20 therapy (red) and treatment with immunosuppressants other than anti-CD20 therapy (yellow). IMID = immune-mediated inflammatory disease. ISP = immunosuppressant.
Disclosures: L. Boekel, None; Y. Besten, None; F. Hooijberg, None; R. Wartena, None; M. Steenhuis, None; E. Vogelzang, None; M. Leeuw, None; S. Atiqi, None; S. Tas, None; W. Lems, None; M. van Ham, None; F. Eftimov, None; E. Stalman, None; L. Wieske, None; T. Kuijpers, None; A. Voskuyl, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; M. Gerritsen, None; C. Krieckaert, None; T. Rispens, None; M. Boers, Novartis; M. Nurmohamed, None; G. Wolbink, None.
Background/Purpose: The Omicron (B.1.1.529) variant of SARS-CoV-2 is associated with substantially lower hospitalization rates compared with previous variants of SARS-CoV-2 (i.e., Wildtype, Alpha [B.1.1.7] and Delta [B.1.617.2] variant) in people of the general population, but data in patients with rheumatic immune-mediated inflammatory diseases (IMIDs) are still scarce. Therefore, we compared the severity of Omicron breakthrough infections between patients with rheumatic IMIDs and healthy controls using data from a large ongoing prospective cohort study.
Methods: In April 2020, all adult patients with rheumatic IMIDs from the Amsterdam Rheumatology and Immunology Center (ARC) in the Netherlands were invited to participate. Patients were asked to recruit their own control participant of the same sex and comparable age. Data on SARS-CoV-2 infections were collected between April 2020 and April 2022 using digital questionnaires. Serum samples were collected during the entire follow-up period and analyzed for the presence of SARS-CoV-2 specific antibodies. Vaccination-naïve SARS-CoV-2 infections were defined as PCR- or serological confirmed infections before SARS-CoV-2 vaccination. As all data on vaccination-naïve infections were collected before the emergence of the Delta variant in the Netherlands, these infections were considered Wildtype or Alpha variant infections. Breakthrough SARS-CoV-2 infections were defined as PCR- or antigen confirmed infections detected at least 14 days after SARS-CoV-2 vaccination. Breakthrough infections were classified as Delta or Omicron infections based on publicly available data on the predominant circulating SARS-CoV-2 variant in the Netherlands. Logistic regression and Fisher's exact test were used for statistical analyses.
Results: In total, 1882 consecutive patients with rheumatic IMIDs and 706 healthy controls were included for analyses on Omicron breakthrough infections. Omicron breakthrough infections were detected in 431 (23%) of 1882 IMID patients and 210 (30%) of 706 healthy controls. Hospitalization was required in 5 (1.2%) of 431 patients and in 1 (0.5%) of 210 controls (aOR: 1.5, 95% CI: 0.2 – 13, P 0.7; Table 1). Anti-CD20 therapy was significantly associated with a higher risk of hospitalization or death (P < 0.001). Hospitalization rates of Omicron breakthrough infections in patients with rheumatic IMIDs were lower compared to Wildtype/Alpha vaccination-naïve infections (aOR: 0.2, 95% CI: 0.06 – 0.5, P < 0.001) and Delta breakthrough infections (aOR: 0.08, 95% CI: 0.02 – 0.3, P < 0.001) (Figure 1 and Table 1). Lastly, a history of Wildtype/Alpha vaccination naïve infections, Delta breakthrough infections and third SARS-CoV-2 vaccine doses were associated with a lower risk of Omicron breakthrough infections (Table 1).
Conclusion: We showed that the risk of a severe disease course of Omicron breakthrough infections is substantially lower compared with previous SARS-CoV-2 variants, and that the risk of severe disease is comparable for most patients with rheumatic IMIDs and healthy controls. However, patients receiving anti-CD20 therapy remain at increased risk of severe COVID-19, even when infected with the Omicron variant of SARS-CoV-2.
 Figure 3. Hospitalization rates of SARS-CoV-2 infections.
Figure 3. Hospitalization rates of SARS-CoV-2 infections. Figure showing the proportion of patients with rheumatic IMIDs (orange) and healthy controls (green) who required hospitalization after infection with SARS-CoV-2 Wildtype/Alpha variant before vaccination (left panel), SARS-CoV-2 Delta variant after vaccination (middle panel), and SARS-CoV-2 Omicron variant after vaccination (right panel). Patients were further divided into subgroups based on immunosuppressive treatment: no immunosuppressive treatment (blue), treatment with anti-CD20 therapy (red) and treatment with immunosuppressants other than anti-CD20 therapy (yellow). IMID = immune-mediated inflammatory disease. ISP = immunosuppressant.

Disclosures: L. Boekel, None; Y. Besten, None; F. Hooijberg, None; R. Wartena, None; M. Steenhuis, None; E. Vogelzang, None; M. Leeuw, None; S. Atiqi, None; S. Tas, None; W. Lems, None; M. van Ham, None; F. Eftimov, None; E. Stalman, None; L. Wieske, None; T. Kuijpers, None; A. Voskuyl, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; M. Gerritsen, None; C. Krieckaert, None; T. Rispens, None; M. Boers, Novartis; M. Nurmohamed, None; G. Wolbink, None.

