Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0914: The Impact of Autoantibodies (RF and ACPA) on the Efficacy of Biological Disease-modifying Antirheumatic Drugs in Rheumatoid Arthritis: Meta-analysis of Randomized Controlled Trials
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- KT
Kaoru Takase-Minegishi, MD, PhD
Yokohama City University Graduate School of Medicine
Yokohama, Japan
Abstract Poster Presenter(s)
Kaoru Takase-Minegishi1, Stephan Böhringer2, Jackie Nam3, Yuko Kaneko4, Frank Behrens5, Saedis Saevarsdottir6, Jacqueline Detert7, Marjatta Leirisalo-Repo8, Désirée van der Heijde9, Robert Landewé10, Sofia Ramiro11 and Diane van der Woude12, 1Department of Hematology and Clinical Immunology, Yokohama City University School of Medicine, Yokohama, Japan, 2Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, Leiden, Netherlands, 3Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, United Kingdom, 4Keio University School of Medicine, Tokyo, Japan, 5CIRI/Rheumatology and Fraunhofer Institute, Translational Medicine and Pharmacology ITMP, Goethe University, Frankfurt, Germany, 6Division of Clinical Epidemiology, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden and Faculty of Medicine, University of Iceland, Stockholm, Sweden, 7Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin Berlin, Berlin, Germany, 8Department of Rheumatology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 9Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Netherlands, 10Amsterdam University Medical Center, Meerssen, Netherlands, 11Leiden University Medical Center, Leiden, Netherlands, 12Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands
Background/Purpose: The impact of autoantibodies on the efficacy of bDMARDs in patients with rheumatoid arthritis (RA) is not yet clear. Despite the fact that this information has been collected by several randomized controlled trials (RCTs), efficacy data for seropositive and seronegative patients separately have generally not been published. Our aim was to comprehensively investigate the efficacy of bDMARDs in patients with RA with RF and/or ACPA compared to patients without these autoantibodies.
Methods: Previous systematic literature reviews performed by EULAR RA management task forces were searched for relevant RCTs published before February 2016 1-3. RCTs including both autoantibody-positive (≤80% of total population) and -negative RA patients were eligible. We contacted authors and/or sponsors of RCTs to report aggregate results from analyses of individual patient data on clinical efficacy outcomes stratified for the presence of autoantibodies (RF+ vs RF- and ACPA+ vs ACPA-). Per trial, relative risks (RR) or mean differences comparing two groups (RF+ vs RF- and ACPA+ vs ACPA-) were calculated for various outcomes (ACR 20/50/70, DAS28 remission, delta DAS28, delta HAQ and radiographic progression) at the timing of the primary endpoint for the bDMARD-arm and the non-bDMARD-arm separately. Subsequently, relative risk ratios (RRRs) were computed, as the ratio of RR of the bDMARD-arm and the RR from the non-bDMARD-treated arm, reflecting whether seropositivity preferentially affected treatment response to bDMARD therapy. A meta-analysis was conducted using a mixed-effect meta-regression in subgroups of patients according to baseline autoantibody status.
Results: Data from 28 eligible RCTs were analyzed: 6 including csDMARD-naïve patients, 14 including csDMARD-inadequate responders (csDMARD-IR), 3 including tumor necrosis factor inhibitor (TNFi)-IR patients and 5 with miscellaneous trial design (e.g. head-to-head trial). In csDMARD-naïve and csDMARD-IR, seropositivity was not associated with a better response to bDMARDs compared to non-bDMARDs. Pooled RRRs of 6-month ACR20 response were 1.02 (0.88-1.18) and 1.09 (0.90-1.32), respectively (Figure 1A and B). Other outcomes followed the same pattern, with no difference between the groups. In TNFi-IR patients, based on 3 trials, the RRR of ACR20 at 6 months was 2.28 (1.31-3.95) (Figure 1C), favouring efficacy in seropositive patients. Other outcomes showed a similar effect, though with large confidence intervals and several reflecting a non-significant difference between the groups (Table 1).
Conclusion: In csDMARD-naïve and csDMARD-IR patients, autoantibodies did not have an impact on the efficacy of bDMARDs in RA. In TNFi-IR patients, there is a possible higher efficacy of bDMARDs in the seropositive group, but the low number of trials, large confidence intervals and inconsistent results across outcomes ask for caution in the interpretation. Seronegative TNF-IR patients may have very heterogenous underlying pathophysiological mechanisms, with a lower probability of good treatment response. Overall, in less treatment-resistant patients, the presence of autoantibodies was not associated with the treatment effect of bDMARDs.
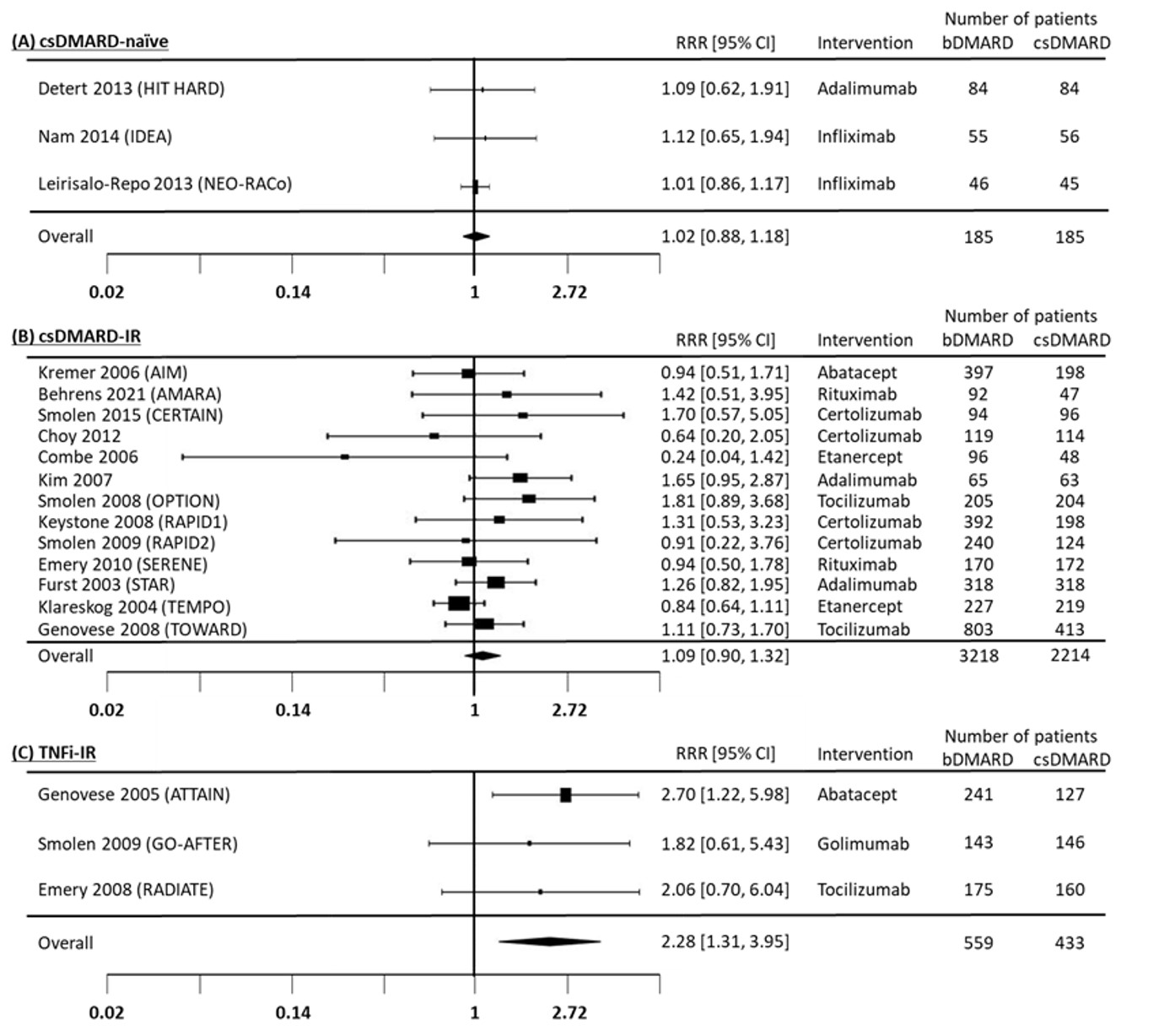 Figure 1. Forest plot for the relative risk ratio for ACR20 at 6 months comparing RF(+) / RF(-) in bDMARD+csDMARD vs RF(+) / RF(-) in csDMARD.
Figure 1. Forest plot for the relative risk ratio for ACR20 at 6 months comparing RF(+) / RF(-) in bDMARD+csDMARD vs RF(+) / RF(-) in csDMARD.
.jpg) Table 1. Pooled outcomes in seropositive (RF+) vs seronegative (RF-) TNFi-IR patients at 6 months.
Table 1. Pooled outcomes in seropositive (RF+) vs seronegative (RF-) TNFi-IR patients at 6 months.
Disclosures: K. Takase-Minegishi, Gilead, Daiichi Sankyo, Mitsubishi Tanabe; S. Böhringer, None; J. Nam, None; Y. Kaneko, Chugai; F. Behrens, AbbVie, Pfizer, Roche, Amgen, Chugai, Prophylix, Novartis, Boehringer, UCB, Bristol Myers Squibb, Celgene, MSD, Biotest, Janssen, Genzyme, Lilly, Sandoz, Sanofi; S. Saevarsdottir, deCODE genetics; J. Detert, None; M. Leirisalo-Repo, None; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Woude, Galapagos.
Background/Purpose: The impact of autoantibodies on the efficacy of bDMARDs in patients with rheumatoid arthritis (RA) is not yet clear. Despite the fact that this information has been collected by several randomized controlled trials (RCTs), efficacy data for seropositive and seronegative patients separately have generally not been published. Our aim was to comprehensively investigate the efficacy of bDMARDs in patients with RA with RF and/or ACPA compared to patients without these autoantibodies.
Methods: Previous systematic literature reviews performed by EULAR RA management task forces were searched for relevant RCTs published before February 2016 1-3. RCTs including both autoantibody-positive (≤80% of total population) and -negative RA patients were eligible. We contacted authors and/or sponsors of RCTs to report aggregate results from analyses of individual patient data on clinical efficacy outcomes stratified for the presence of autoantibodies (RF+ vs RF- and ACPA+ vs ACPA-). Per trial, relative risks (RR) or mean differences comparing two groups (RF+ vs RF- and ACPA+ vs ACPA-) were calculated for various outcomes (ACR 20/50/70, DAS28 remission, delta DAS28, delta HAQ and radiographic progression) at the timing of the primary endpoint for the bDMARD-arm and the non-bDMARD-arm separately. Subsequently, relative risk ratios (RRRs) were computed, as the ratio of RR of the bDMARD-arm and the RR from the non-bDMARD-treated arm, reflecting whether seropositivity preferentially affected treatment response to bDMARD therapy. A meta-analysis was conducted using a mixed-effect meta-regression in subgroups of patients according to baseline autoantibody status.
Results: Data from 28 eligible RCTs were analyzed: 6 including csDMARD-naïve patients, 14 including csDMARD-inadequate responders (csDMARD-IR), 3 including tumor necrosis factor inhibitor (TNFi)-IR patients and 5 with miscellaneous trial design (e.g. head-to-head trial). In csDMARD-naïve and csDMARD-IR, seropositivity was not associated with a better response to bDMARDs compared to non-bDMARDs. Pooled RRRs of 6-month ACR20 response were 1.02 (0.88-1.18) and 1.09 (0.90-1.32), respectively (Figure 1A and B). Other outcomes followed the same pattern, with no difference between the groups. In TNFi-IR patients, based on 3 trials, the RRR of ACR20 at 6 months was 2.28 (1.31-3.95) (Figure 1C), favouring efficacy in seropositive patients. Other outcomes showed a similar effect, though with large confidence intervals and several reflecting a non-significant difference between the groups (Table 1).
Conclusion: In csDMARD-naïve and csDMARD-IR patients, autoantibodies did not have an impact on the efficacy of bDMARDs in RA. In TNFi-IR patients, there is a possible higher efficacy of bDMARDs in the seropositive group, but the low number of trials, large confidence intervals and inconsistent results across outcomes ask for caution in the interpretation. Seronegative TNF-IR patients may have very heterogenous underlying pathophysiological mechanisms, with a lower probability of good treatment response. Overall, in less treatment-resistant patients, the presence of autoantibodies was not associated with the treatment effect of bDMARDs.
 Figure 1. Forest plot for the relative risk ratio for ACR20 at 6 months comparing RF(+) / RF(-) in bDMARD+csDMARD vs RF(+) / RF(-) in csDMARD.
Figure 1. Forest plot for the relative risk ratio for ACR20 at 6 months comparing RF(+) / RF(-) in bDMARD+csDMARD vs RF(+) / RF(-) in csDMARD..jpg) Table 1. Pooled outcomes in seropositive (RF+) vs seronegative (RF-) TNFi-IR patients at 6 months.
Table 1. Pooled outcomes in seropositive (RF+) vs seronegative (RF-) TNFi-IR patients at 6 months.Disclosures: K. Takase-Minegishi, Gilead, Daiichi Sankyo, Mitsubishi Tanabe; S. Böhringer, None; J. Nam, None; Y. Kaneko, Chugai; F. Behrens, AbbVie, Pfizer, Roche, Amgen, Chugai, Prophylix, Novartis, Boehringer, UCB, Bristol Myers Squibb, Celgene, MSD, Biotest, Janssen, Genzyme, Lilly, Sandoz, Sanofi; S. Saevarsdottir, deCODE genetics; J. Detert, None; M. Leirisalo-Repo, None; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Woude, Galapagos.

