Back
Poster Session B
Session: (0807–0832) Miscellaneous Rheumatic and Inflammatory Diseases Poster II
0808: Transitional Care in Juvenile Idiopathic Arthritis: Timing, Prevalence of Subtypes and Treatment Profile in a Spanish Tertiary Hospital
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CP
Cristobal Pavez, Sr, MD
Hospital Universitario y Politecnico La Fe
Valencia, Spain
Abstract Poster Presenter(s)
Cristobal Pavez Perales1, Alba Torrat Novés2, José Ivorra Cortés1, Samuel Leal Rodriguez1, Anderson V Huaylla Quispe3, Carmen Riesco Bárcena2, Laura Mas Sanchez1, Pablo Muñoz Martinez4, Elena Grau García1, Elvira Vicens Bernabeu1, José Eloy Oller Rodrígez1, Francisco Miguel Ortiz Sanjuan5, Isabel Martínez-Cordellat1, Rosa Negueroles Albuixech1, Berta Lopez Montecinos6, Carmen Nájera Herranz1, Inés Cánovas Olmos1, Daniel Ramos Castro1, Inmaculada Calvo Penades7, Luis González Puig8 and José andrés Román ivorra9, 1Rheumatology Department. Hospital Universitario y Politécnico La Fe, València, Spain, 2Rheumatology Department. Hospital Universitario y Politécnico La Fe, València, 3Rheumatology Department. Hospital Universitario y Politécnico La Fe, València, Comunidad Valenciana, Spain, 4Rheumatology Department. Hospital Universitario y Politécnico La Fe, Sagunto, Spain, 5Rheumatology Department. Hospital Universitario y Politécnico La Fe, Spain, Spain, 6Pediatric Rheumatology Unit. Hospital Universitario y Politécnico La Fe, València, Comunidad Valenciana, Spain, 7Pediatric Rheumatology Unit, Hospital Universitario y Politécnico La Fe, València, Spain, 8Rheumatology Department. Hospital Universitario y Politécnico La Fe, Torrente, Valencia, Spain, 9Hospital Universitari i Politécnic la Fe, València, Spain
Background/Purpose: Juvenile Idiopathic Arthritis (JIA) is the leading cause of chronic inflammatory rheumatic disease in children. It's classified into subtypes with different relative prevalences depending on geographical area (Oligarticular subtype predominates in Western Europe/North America. Enthesitis-related arthritis subtype predominates in Eastern Europe/Asia). To ensure continuity of care in adult rheumatology services, a systematic transition process is recommended. Various authors recommend that the process, of which pediatric and adult rheumatology teams should be part, begins around 14 years and ends around 18 years of age. We aim to study the age, relative prevalence, and treatment profile in JIA subtypes at the beginning of the transitional care.
Methods: Descriptive and cross-sectional study of patients with JIA (according to ILAR criteria), diagnosed and treated in the pediatric rheumatology service and seen in the transitional care unit of the adult rheumatology service within the same tertiary hospital between January 2013 and December 2018. Demographic, clinical, analytical, and treatment data were collected at the first visit to the transitional care unit.
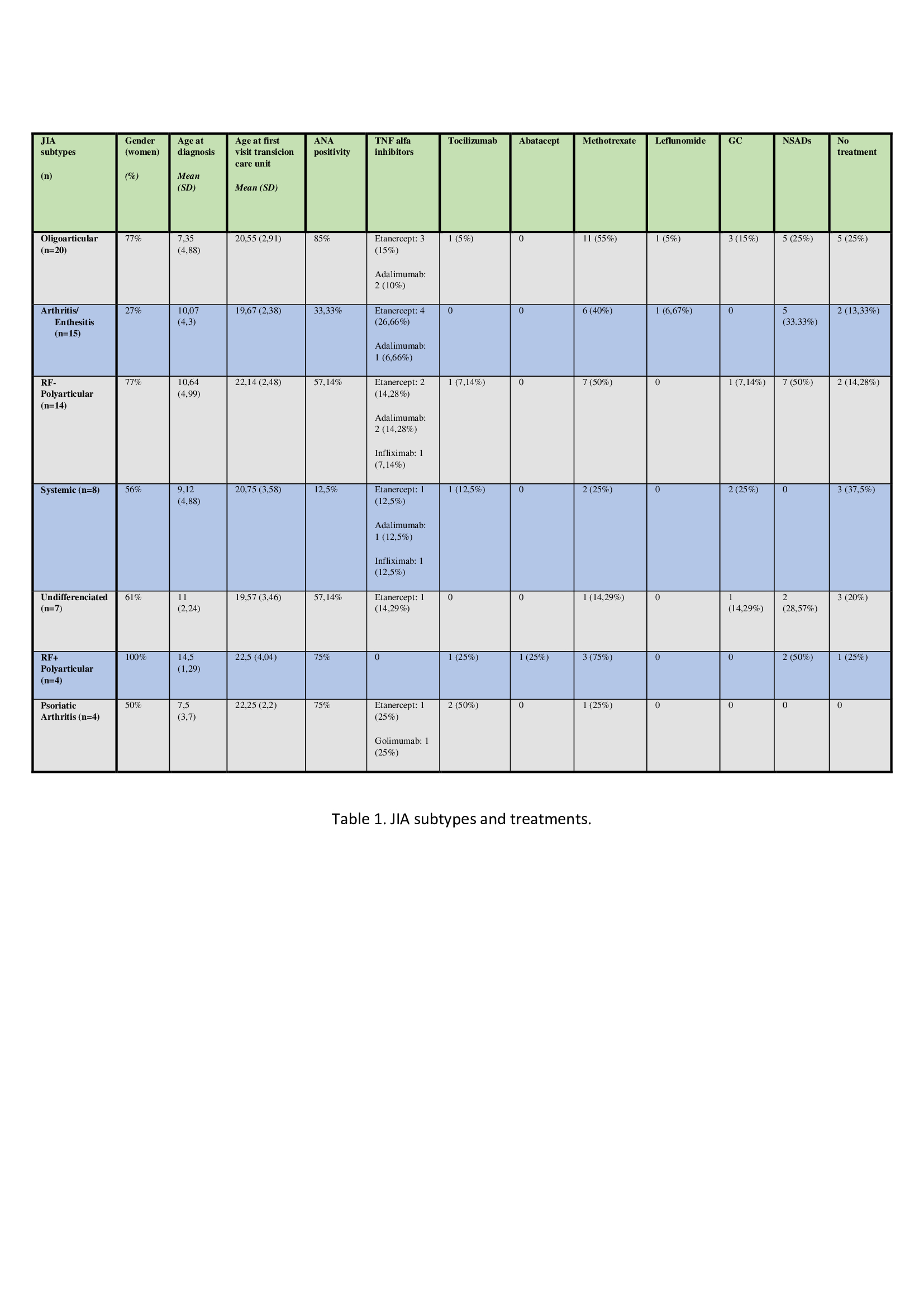
Results: 72 patients were included (46 women), mean age at diagnosis of 9.5 ± 4.6y and a mean of 11.3 ± 4.36y from diagnosis to first visit at the transitional care unit. 27.7% were diagnosed with oligoarticular JIA, 20.8% with arthritis-enthesitis JIA, 19.4% with Rheumatoid Factor negative (RF-) polyarticular JIA, 11.1% with systemic JIA, 9.7% with undifferentiated JIA, 5.5% with Rheumatoid Factor positive (RF+) polyarticular JIA and 5.5% of psoriatic arthritis. The mean age at the first visit to the transitional care unit was 20.81 ± 2.96y (no differences between subtypes). Oral ulcers (20.8%), anterior uveitis (13.8%), and enthesitis (13.8%) were the most frequent extra-articular manifestations. 56.9% had antinuclear antibodies (ANA) titers >1/160 at some point in course of the disease. 43% were treated with methotrexate, 38% with biological therapies, 11.% with glucocorticoids (GC) and 22.% had no treatment (Table).
Conclusion: The oligoarticular form was the most prevalent subtype of JIA, similar to previously published series from Western Europe. The first visit at the transition care unit occurred significantly later than recommended by various authors. The most frequent treatment was methotrexate. The use of biological therapies was high, with TNF alpha inhibitors being the most widely used, especially etanercept.
Disclosures: C. Pavez Perales, None; A. Torrat Novés, None; J. Ivorra Cortés, None; S. Leal Rodriguez, None; A. Huaylla Quispe, None; C. Riesco Bárcena, None; L. Mas Sanchez, None; P. Muñoz Martinez, None; E. Grau García, None; E. Vicens Bernabeu, None; J. Oller Rodrígez, None; F. Ortiz Sanjuan, None; I. Martínez-Cordellat, None; R. Negueroles Albuixech, None; B. Lopez Montecinos, Novartis; C. Nájera Herranz, None; I. Cánovas Olmos, None; D. Ramos Castro, None; I. Calvo Penades, Novartis, GlaxoSmithKlein(GSK), Ipsen, AbbVie/Abbott, Sobi; L. González Puig, None; J. Román ivorra, None.
Background/Purpose: Juvenile Idiopathic Arthritis (JIA) is the leading cause of chronic inflammatory rheumatic disease in children. It's classified into subtypes with different relative prevalences depending on geographical area (Oligarticular subtype predominates in Western Europe/North America. Enthesitis-related arthritis subtype predominates in Eastern Europe/Asia). To ensure continuity of care in adult rheumatology services, a systematic transition process is recommended. Various authors recommend that the process, of which pediatric and adult rheumatology teams should be part, begins around 14 years and ends around 18 years of age. We aim to study the age, relative prevalence, and treatment profile in JIA subtypes at the beginning of the transitional care.
Methods: Descriptive and cross-sectional study of patients with JIA (according to ILAR criteria), diagnosed and treated in the pediatric rheumatology service and seen in the transitional care unit of the adult rheumatology service within the same tertiary hospital between January 2013 and December 2018. Demographic, clinical, analytical, and treatment data were collected at the first visit to the transitional care unit.
Results: 72 patients were included (46 women), mean age at diagnosis of 9.5 ± 4.6y and a mean of 11.3 ± 4.36y from diagnosis to first visit at the transitional care unit. 27.7% were diagnosed with oligoarticular JIA, 20.8% with arthritis-enthesitis JIA, 19.4% with Rheumatoid Factor negative (RF-) polyarticular JIA, 11.1% with systemic JIA, 9.7% with undifferentiated JIA, 5.5% with Rheumatoid Factor positive (RF+) polyarticular JIA and 5.5% of psoriatic arthritis. The mean age at the first visit to the transitional care unit was 20.81 ± 2.96y (no differences between subtypes). Oral ulcers (20.8%), anterior uveitis (13.8%), and enthesitis (13.8%) were the most frequent extra-articular manifestations. 56.9% had antinuclear antibodies (ANA) titers >1/160 at some point in course of the disease. 43% were treated with methotrexate, 38% with biological therapies, 11.% with glucocorticoids (GC) and 22.% had no treatment (Table).
Conclusion: The oligoarticular form was the most prevalent subtype of JIA, similar to previously published series from Western Europe. The first visit at the transition care unit occurred significantly later than recommended by various authors. The most frequent treatment was methotrexate. The use of biological therapies was high, with TNF alpha inhibitors being the most widely used, especially etanercept.

Disclosures: C. Pavez Perales, None; A. Torrat Novés, None; J. Ivorra Cortés, None; S. Leal Rodriguez, None; A. Huaylla Quispe, None; C. Riesco Bárcena, None; L. Mas Sanchez, None; P. Muñoz Martinez, None; E. Grau García, None; E. Vicens Bernabeu, None; J. Oller Rodrígez, None; F. Ortiz Sanjuan, None; I. Martínez-Cordellat, None; R. Negueroles Albuixech, None; B. Lopez Montecinos, Novartis; C. Nájera Herranz, None; I. Cánovas Olmos, None; D. Ramos Castro, None; I. Calvo Penades, Novartis, GlaxoSmithKlein(GSK), Ipsen, AbbVie/Abbott, Sobi; L. González Puig, None; J. Román ivorra, None.

