Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0724–0751) Health Services Research Poster II
0729: Understanding the Practice and Process of Patient Reported Outcome Measures Collection in North American Pediatric Rheumatology Clinics: A Survey of the Pediatric Rheumatology-Care and Outcomes Improvement Network
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- YG
Y. Ingrid Goh, PhD
The Hospital for Sick Children
Toronto, ON, Canada
Abstract Poster Presenter(s)
Y. Ingrid Goh1, Esi Morgan2, Meghan Ryan3, Beth Gottlieb4 and Nancy Pan5, 1Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute, Toronto, ON, Canada, 2Seattle Children's Hospital, Seattle, WA, 3University of Minnesota, Vadnais Heights, MN, 4Cohen Children's Medical Center, Lake Success, NY, 5Hospital for Special Surgery, New York, NY
Background/Purpose: Patients/proxies (Pts) complete patient reported outcome measures (PROMs) to inform their healthcare team about their health status. PROMs completed by Pts prior to their appointment assist their healthcare providers to prepare for their upcoming visit. Since there are many validated PROMs, knowing which instruments are used allows for the comparison of PROMs across institutions. We aimed to a) determine what PROMs are being completed, b) understand the operational process to completing PROMs, and c) understand the facilitators and barriers to collecting PROMs.
Methods: A REDCap survey was sent to the lead representative for each Pediatric Rheumatology-Care and Outcomes Improvement Network (PR-COIN) site. Quantitative data were analyzed using descriptive statistics and qualitative data were thematically analyzed.
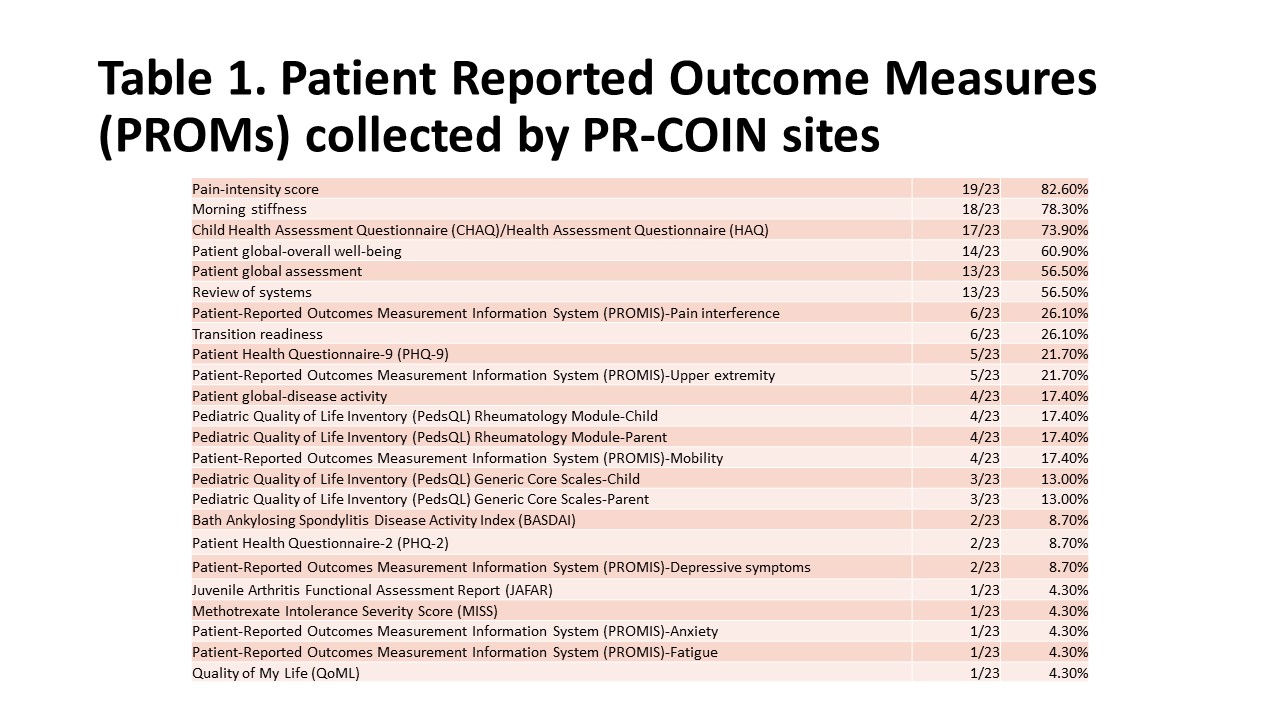
Results: All 23 PR-COIN sites responded to the survey. PROMs are collected by 21/23 (91%) sites (Table 1). Pain intensity score, morning stiffness and the Child Health Assessment Questionnaire (CHAQ) were the top three collected PROMs for clinical and research purposes. Interestingly, the patient global assessment was collected more often for research only purposes rather than clinical only purposes.
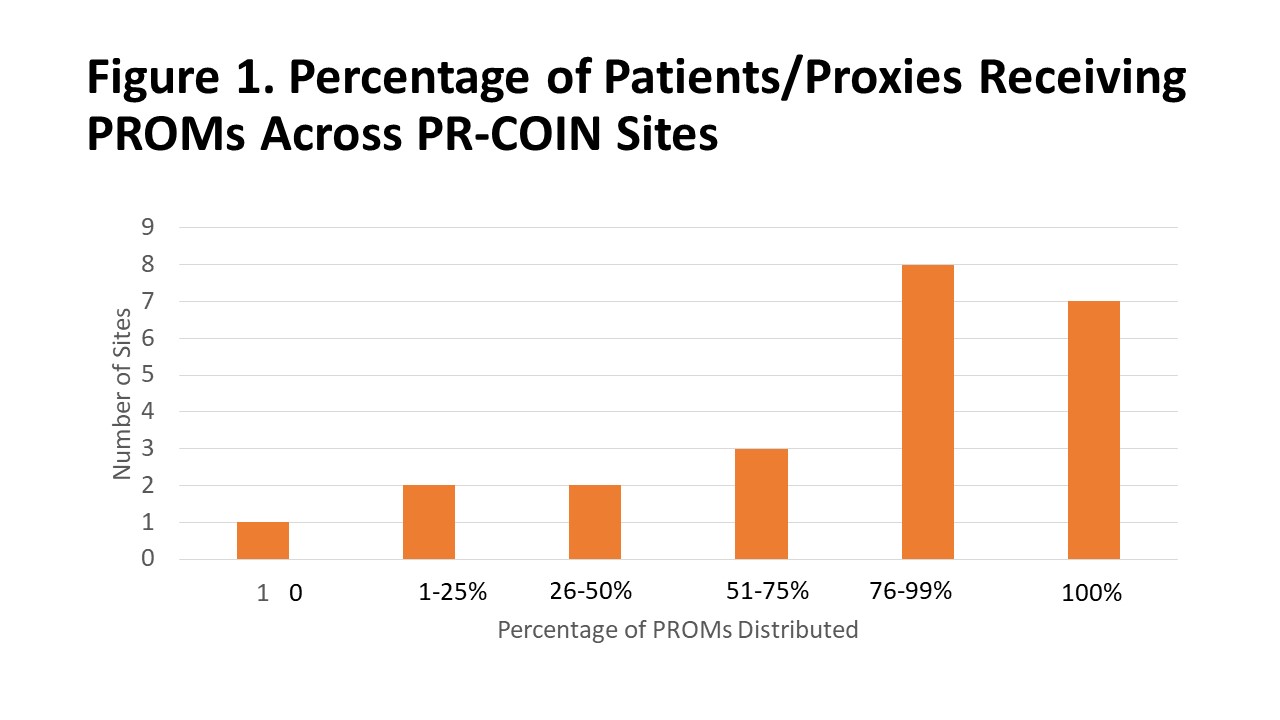
Respondents indicated that PROMs were collected using both paper and/or electronic methods, with the majority of sites collecting PROMs only on paper. Respondents estimated that staff spent three minutes administering PROMs compared to six minutes for Pts to self-administer. PROMs were administered by clinical, research and front desk staff, as well as volunteers. More than ¾ respondents said Pts could also self-administer PROMs. PROMs were usually completed by either patient or parent mostly before or during the appointment, with more than half of the Pts at each site completing the PROMs (Figure 1).
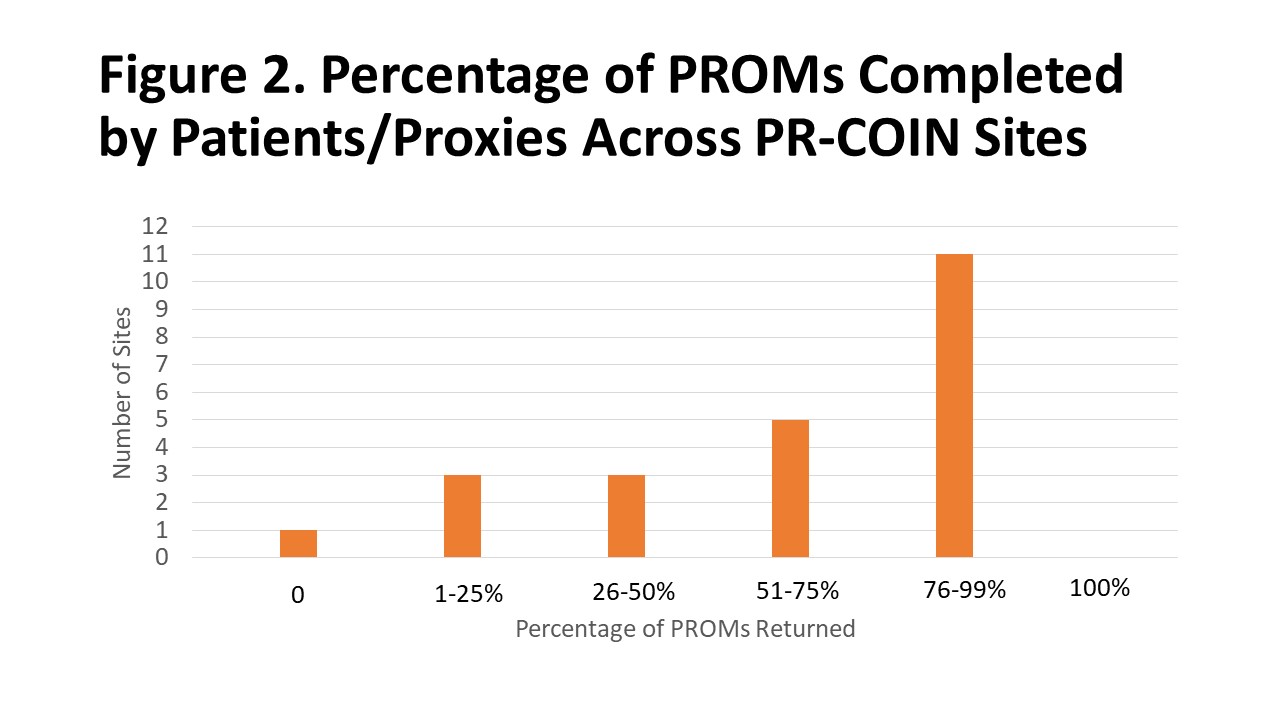
PROMs were manually scored at most sites by clinical or research staff, or volunteers. Respondents estimated that clinical or research staff took about four minutes to enter scores into the electronic medical records (EMR). Although some sites reported their Pts could complete their PROMs electronically, 5/12 (42%) sites reported that scores could not automatically be imported into the patient's EMR.
Respondents indicated that availability of human resources, training and culture were the greatest facilitators, whereas limited time and lack of staff were the greatest barriers to PROMs completion.
Conclusion: Pts complete a variety of PROMs either by paper or electronically. The process of administering and scoring of PROMs vary across sites. Electronic completion with direct upload into the EMR could improve the PROMs collection process, as would creating a culture that is supported by trained staff. The alignment of PROMs and the collection of responses using similar scales would facilitate the network's ability to compare patient reported outcomes. The results from this survey informed recent updates to the PR-COIN registry's data fields.
Disclosures: Y. Goh, None; E. Morgan, None; M. Ryan, None; B. Gottlieb, None; N. Pan, None.
Background/Purpose: Patients/proxies (Pts) complete patient reported outcome measures (PROMs) to inform their healthcare team about their health status. PROMs completed by Pts prior to their appointment assist their healthcare providers to prepare for their upcoming visit. Since there are many validated PROMs, knowing which instruments are used allows for the comparison of PROMs across institutions. We aimed to a) determine what PROMs are being completed, b) understand the operational process to completing PROMs, and c) understand the facilitators and barriers to collecting PROMs.
Methods: A REDCap survey was sent to the lead representative for each Pediatric Rheumatology-Care and Outcomes Improvement Network (PR-COIN) site. Quantitative data were analyzed using descriptive statistics and qualitative data were thematically analyzed.
Results: All 23 PR-COIN sites responded to the survey. PROMs are collected by 21/23 (91%) sites (Table 1). Pain intensity score, morning stiffness and the Child Health Assessment Questionnaire (CHAQ) were the top three collected PROMs for clinical and research purposes. Interestingly, the patient global assessment was collected more often for research only purposes rather than clinical only purposes.
Respondents indicated that PROMs were collected using both paper and/or electronic methods, with the majority of sites collecting PROMs only on paper. Respondents estimated that staff spent three minutes administering PROMs compared to six minutes for Pts to self-administer. PROMs were administered by clinical, research and front desk staff, as well as volunteers. More than ¾ respondents said Pts could also self-administer PROMs. PROMs were usually completed by either patient or parent mostly before or during the appointment, with more than half of the Pts at each site completing the PROMs (Figure 1).
PROMs were manually scored at most sites by clinical or research staff, or volunteers. Respondents estimated that clinical or research staff took about four minutes to enter scores into the electronic medical records (EMR). Although some sites reported their Pts could complete their PROMs electronically, 5/12 (42%) sites reported that scores could not automatically be imported into the patient's EMR.
Respondents indicated that availability of human resources, training and culture were the greatest facilitators, whereas limited time and lack of staff were the greatest barriers to PROMs completion.
Conclusion: Pts complete a variety of PROMs either by paper or electronically. The process of administering and scoring of PROMs vary across sites. Electronic completion with direct upload into the EMR could improve the PROMs collection process, as would creating a culture that is supported by trained staff. The alignment of PROMs and the collection of responses using similar scales would facilitate the network's ability to compare patient reported outcomes. The results from this survey informed recent updates to the PR-COIN registry's data fields.



Disclosures: Y. Goh, None; E. Morgan, None; M. Ryan, None; B. Gottlieb, None; N. Pan, None.

