Back
Ignite Talk
Session: Ignite Session 3A
0470: Long-term Efficacy of Tocilizumab Monotherapy After Ultra-short Glucocorticoid Administration to Treat Giant Cell Arteritis – One Year Follow-up of the GUSTO Trial
Sunday, November 13, 2022
9:20 AM – 9:25 AM Eastern Time
Location: Northern Liberties Stage
- LC
Lisa Christ, MD
Inselspital, University Bern, Switzerland
Bern, SwitzerlandDisclosure: Disclosure(s): No relevant disclosure to display
Ignite Speaker(s)
Lisa Christ1, Luca Seitz2, Godehard Scholz1, Lukas Buetikofer3, Florian Kollert1, Stephan Reichenbach4 and Peter Villiger5, 1Department of Rheumatology and Immunology, University of Bern, Inselspital, Bern, Switzerland, 2Department of Rheumatology and Immunology, University of Bern, Inselspital, Switzerland, 3CTU Bern, University of Bern, Bern, Switzerland, 4University of Bern, Institute for Social and Preventive Medicine, Bern, Switzerland, 5Medical Center Monbijou, Rheumatology and Immunology, Bern, Switzerland
Background/Purpose: Two randomised controlled trials (RCT) [1, 2] demonstrated a glucocorticoid (GC)-sparing effect of tocilizumab (TCZ) of at least 50% in the treatment of giant cell arteritis (GCA). The GUSTO (Giant cell arteritis treatment with Ultra-Short glucocorticoids and TOcilizumab) trial was set up to evaluate the efficacy and safety of TCZ-monotherapy after a 3-day GC-pulse in new-onset GCA.
The objectives of this analysis were to explore the maintenance of remission 1 year after discontinuation of TCZ treatment. Data up to week 104 are presented.
Methods: Eighteen patients with newly diagnosed GCA were enrolled in this investigator-initiated, single-arm, single-center, open-label clinical trial [3]. Patients received 500 mg methylprednisolone intravenously for 3 consecutive days. Thereafter, GC treatment was discontinued and TCZ (8 mg/kg bodyweight) was administered intravenously, followed by weekly subcutaneous TCZ injections (162 mg) from day 10 until week 52. Patients in clinical remission stopped TCZ at week 52 and entered the follow-up study. Maintenance of efficacy at week 104 included the proportion of patients with complete relapse-free remission of disease at week 104, and time to first relapse after week 52.
Results: At baseline there were 12/18 female patients, and the median age was 72 (range 67-75) years. Overall, 15/18 complained cranial symptoms (10/18 jaw claudication, 6/18 visual symptoms), 10/18 suffered from polymyalgia rheumatica symptoms, 16/18 had positive cranial ultrasound, and 13/18 had characteristic histopathology. At week 52, 13/18 patients were in relapse-free remission and entered the follow-up study. 1/13 patients presented with a minor relapse at week 72. Remission was achieved after restart of TCZ-monotherapy. At week 104, 12/18 patients were in relapse-free remission.
Conclusion: After a 3-days pulse of methylprednisolone followed by 52 weeks of TCZ monotherapy, drug-free remission was maintained until week 104 in all but one patient entering long-term extension (12/13, 92%). This relapse rate is substantially lower than reported in the RCTs [1,2]. It may – at least in part – be explained by the patient characteristics (exclusively new diagnoses), and potentially by the initial 3-day GC pulse. As a proof-of-concept study, the protocol is not intended to be used in everyday clinical practice.
References
1. Villiger, et al. Lancet, 2016
2. Stone, et al. NEJM, 2017
3. Christ, et al. Lancet Rheumatol, 2021
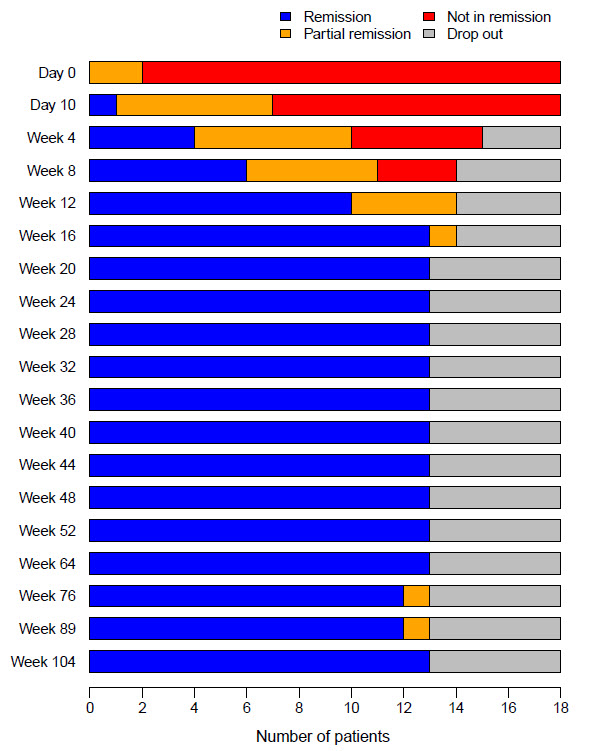 Disease status of patients at each visit (Day 0 – week 104, n=18)
Disease status of patients at each visit (Day 0 – week 104, n=18)
Disclosures: L. Christ, Novartis, Gilead, Roche, Pfizer, Bristol-Myers Squibb(BMS); L. Seitz, None; G. Scholz, None; L. Buetikofer, None; F. Kollert, Roche; S. Reichenbach, None; P. Villiger, Roche, Merck/MSD, AbbVie/Abbott, Pfizer, Novartis, Amgen, Bristol-Myers Squibb(BMS).
Background/Purpose: Two randomised controlled trials (RCT) [1, 2] demonstrated a glucocorticoid (GC)-sparing effect of tocilizumab (TCZ) of at least 50% in the treatment of giant cell arteritis (GCA). The GUSTO (Giant cell arteritis treatment with Ultra-Short glucocorticoids and TOcilizumab) trial was set up to evaluate the efficacy and safety of TCZ-monotherapy after a 3-day GC-pulse in new-onset GCA.
The objectives of this analysis were to explore the maintenance of remission 1 year after discontinuation of TCZ treatment. Data up to week 104 are presented.
Methods: Eighteen patients with newly diagnosed GCA were enrolled in this investigator-initiated, single-arm, single-center, open-label clinical trial [3]. Patients received 500 mg methylprednisolone intravenously for 3 consecutive days. Thereafter, GC treatment was discontinued and TCZ (8 mg/kg bodyweight) was administered intravenously, followed by weekly subcutaneous TCZ injections (162 mg) from day 10 until week 52. Patients in clinical remission stopped TCZ at week 52 and entered the follow-up study. Maintenance of efficacy at week 104 included the proportion of patients with complete relapse-free remission of disease at week 104, and time to first relapse after week 52.
Results: At baseline there were 12/18 female patients, and the median age was 72 (range 67-75) years. Overall, 15/18 complained cranial symptoms (10/18 jaw claudication, 6/18 visual symptoms), 10/18 suffered from polymyalgia rheumatica symptoms, 16/18 had positive cranial ultrasound, and 13/18 had characteristic histopathology. At week 52, 13/18 patients were in relapse-free remission and entered the follow-up study. 1/13 patients presented with a minor relapse at week 72. Remission was achieved after restart of TCZ-monotherapy. At week 104, 12/18 patients were in relapse-free remission.
Conclusion: After a 3-days pulse of methylprednisolone followed by 52 weeks of TCZ monotherapy, drug-free remission was maintained until week 104 in all but one patient entering long-term extension (12/13, 92%). This relapse rate is substantially lower than reported in the RCTs [1,2]. It may – at least in part – be explained by the patient characteristics (exclusively new diagnoses), and potentially by the initial 3-day GC pulse. As a proof-of-concept study, the protocol is not intended to be used in everyday clinical practice.
References
1. Villiger, et al. Lancet, 2016
2. Stone, et al. NEJM, 2017
3. Christ, et al. Lancet Rheumatol, 2021
 Disease status of patients at each visit (Day 0 – week 104, n=18)
Disease status of patients at each visit (Day 0 – week 104, n=18)Disclosures: L. Christ, Novartis, Gilead, Roche, Pfizer, Bristol-Myers Squibb(BMS); L. Seitz, None; G. Scholz, None; L. Buetikofer, None; F. Kollert, Roche; S. Reichenbach, None; P. Villiger, Roche, Merck/MSD, AbbVie/Abbott, Pfizer, Novartis, Amgen, Bristol-Myers Squibb(BMS).

