Back
Ignite Talk
Session: Ignite Session 3C
1459: Evolution of Subjective Cognitive Impairment Overtime in SLE Patients: Bayesian Longitudinal Item Response Theory Modelling
Sunday, November 13, 2022
9:30 AM – 9:35 AM Eastern Time
Location: South Philly Stage
Ignite Speaker(s)
Michelle Barraclough1, Juan Pablo Diaz-Martinez2, Andrea Knight3, Kathleen Bingham4, Jiandong Su2, Mahta Kakvan5, Carolina Munoz2, Maria Carmela Tartaglia6, Leslet Ruttan7, Joan Wither5, May Choi8, Nicole Anderson9, Dennisse Bonilla2, Simone Appenzeller10, Ben Parker11, Patricia Katz12, Dorcas Beaton13, Robin Green7, Ian N. Bruce14 and Zahi Touma2, 1Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, Centre for Epidemiology Versus Arthritis, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Toronto, ON, Canada, 2Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 3The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 4Centre for Mental Health, University Health Network; Department of Psychiatry, University of Toronto, Toronto, ON, Canada, 5Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Toronto, ON, Canada, 6University of Toronto Krembil Neurosciences Centre, Toronto, ON, Canada, 7University Health Network-Toronto Rehabilitation Institute, Toronto, ON, Canada, 8Brigham and Women's Hospital | University of Calgary, Calgary, AB, Canada, 9Schroeder Arthritis Institute, Krembil Research Institute and University Health Network, University of Toronto, Toronto, ON, Canada, 10Unicamp, Campinas, São Paulo, Brazil, 11Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom, 12UCSF, San Rafael, CA, 13Institute for Work & Health, Toronto, ON, Canada, 14Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom
Background/Purpose: Subjective cognitive impairment (SCI) is a significant problem in SLE and there are a lack of studies assessing change over time in SCI. The Perceived Deficits Questionnaire (PDQ-20) is designed to measure SCI. This tool consists of 20 items assessing different perceived cognitive difficulties. Participants respond using a 5-point Likert scale (scale categories from 0-never to 4-almost always experiencing the difficulty). Using the PDQ-20, the aim of this study was to assess SCI changes over time in an SLE cohort. In addition, the usefulness of each PDQ-20 item as an SCI measure was explored.
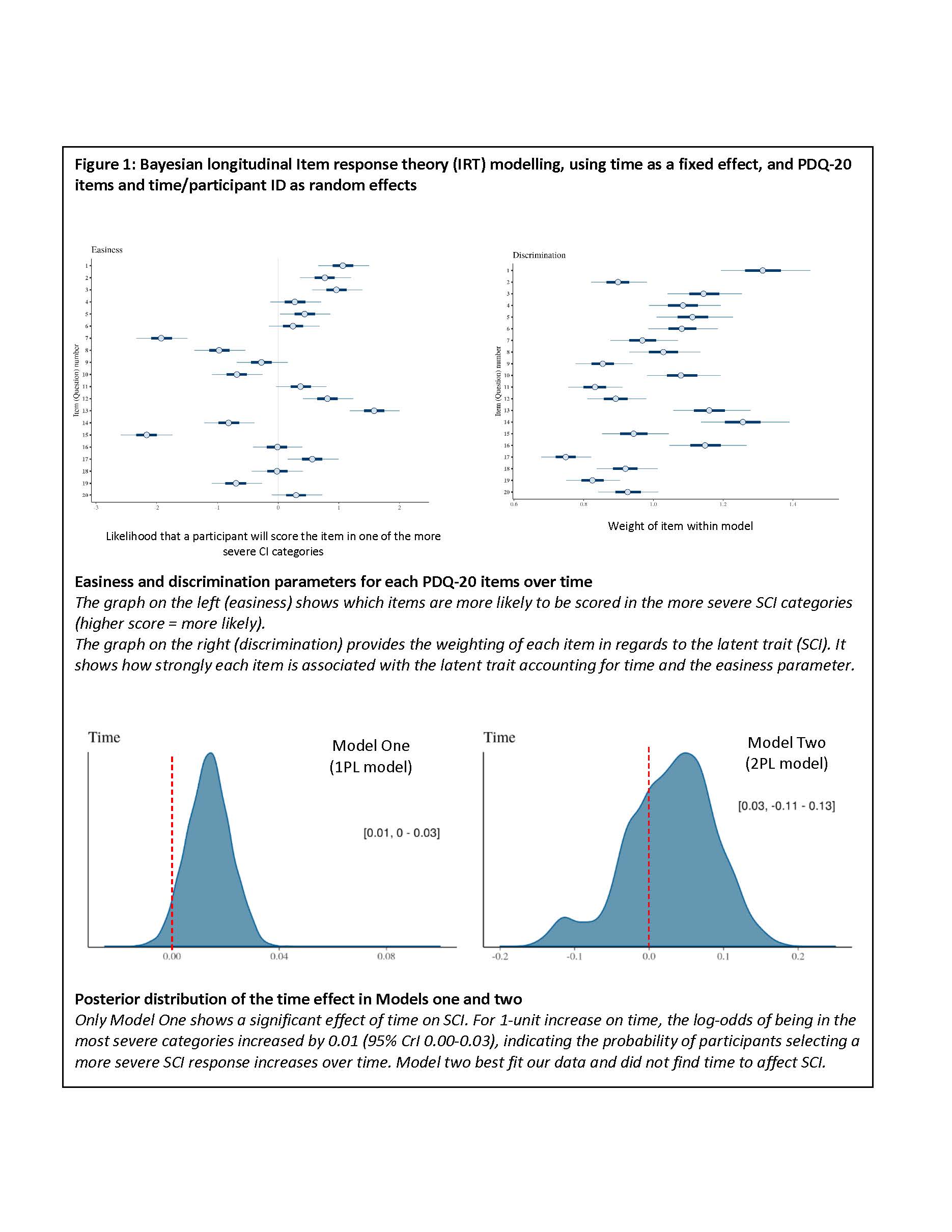
Methods: Demographic, clinical, and psychiatric data, patient reported outcomes, including the PDQ-20 and the ACR neuropsychological battery were collected at multiple visits (0, 6, 12 and 24 months) for participants meeting the 2019 EULAR/ACR classification criteria for SLE. Bayesian longitudinal item response theory (IRT) modelling - using time as a fixed effect, and PDQ-20 items and time/participant as random effects - were undertaken to assess SCI changes over time. Model One was fitted with an easiness parameter that was estimated for each PDQ-20 item (higher values represent a higher probability of participants selecting a more severe SCI category). Model Two repeated model one, but also included a discrimination parameter (DP) per item. The DP represented the weight of each PDQ-20 item within the latent trait (SCI). Finally, leave-one-out cross-validation (loo-cv) was used to determine the best statistical model for our data set.
Results: Results from 283 participants for 612 visits were used. Participants were representative of a typical SLE patient group (Table 1). The easiness parameters for each PDQ-20 item showed items 1, 3, 7, 13, and 15 had higher positive values, meaning the chances of selecting one of the more severe SCI categories was increased for these items. Model One showed that the log-odds of SCI increases over time (0.01 95% CrI 0.00-0.03). Model Two indicated that items 1, 14, 17, and 19 held the most discrimination, meaning that these items can distinguish between patients with very similar latent abilities, but the effect of time was no longer significant (0.03 95% CrI -0.11-0.13) (Figure 1). Loo-cv showed model two to be the best fit for our data. Finally, plotting the probability of selecting each PDQ-20 SCI category over time found that the probability of selecting the more severe categories (2-4) increased over time, whereas the less severe (0-1) reduced over time (Figure 2).
Conclusion: This analysis indicated that SCI may increase over time. It also highlighted which items on the PDQ-20 are more likely to be scored with a high SCI and which are more discriminate with regards to the latent trait in our SLE cohort. This complex analysis is the first to have used IRT for the assessment of longitudinal Likert data and did so by fitting the data to one trajectory (worsening SCI over time). Our future analyses will use this new technique to investigate multiple trajectories of SCI over time within our SLE cohort.
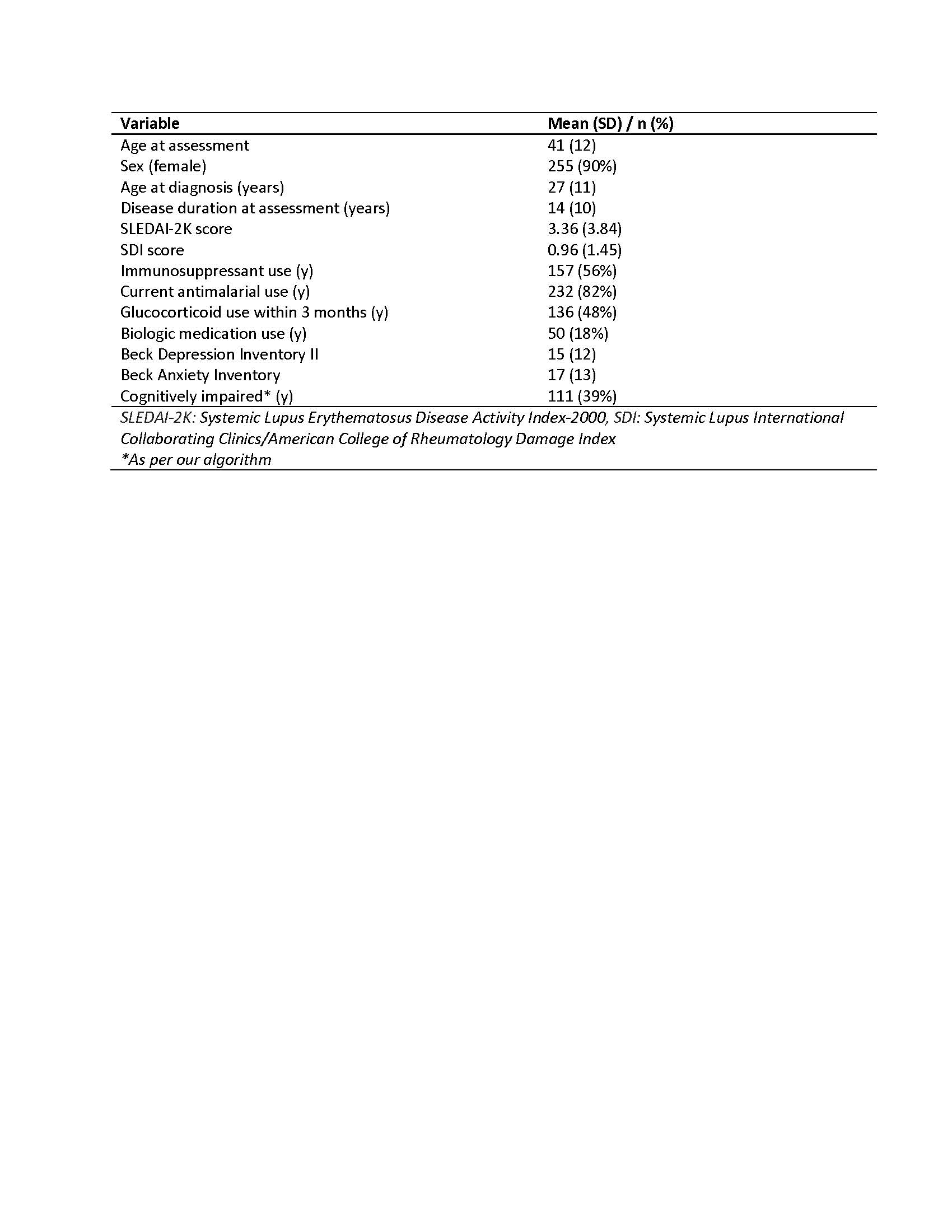 Table 1. Baseline characteristics of the study cohort
Table 1. Baseline characteristics of the study cohort
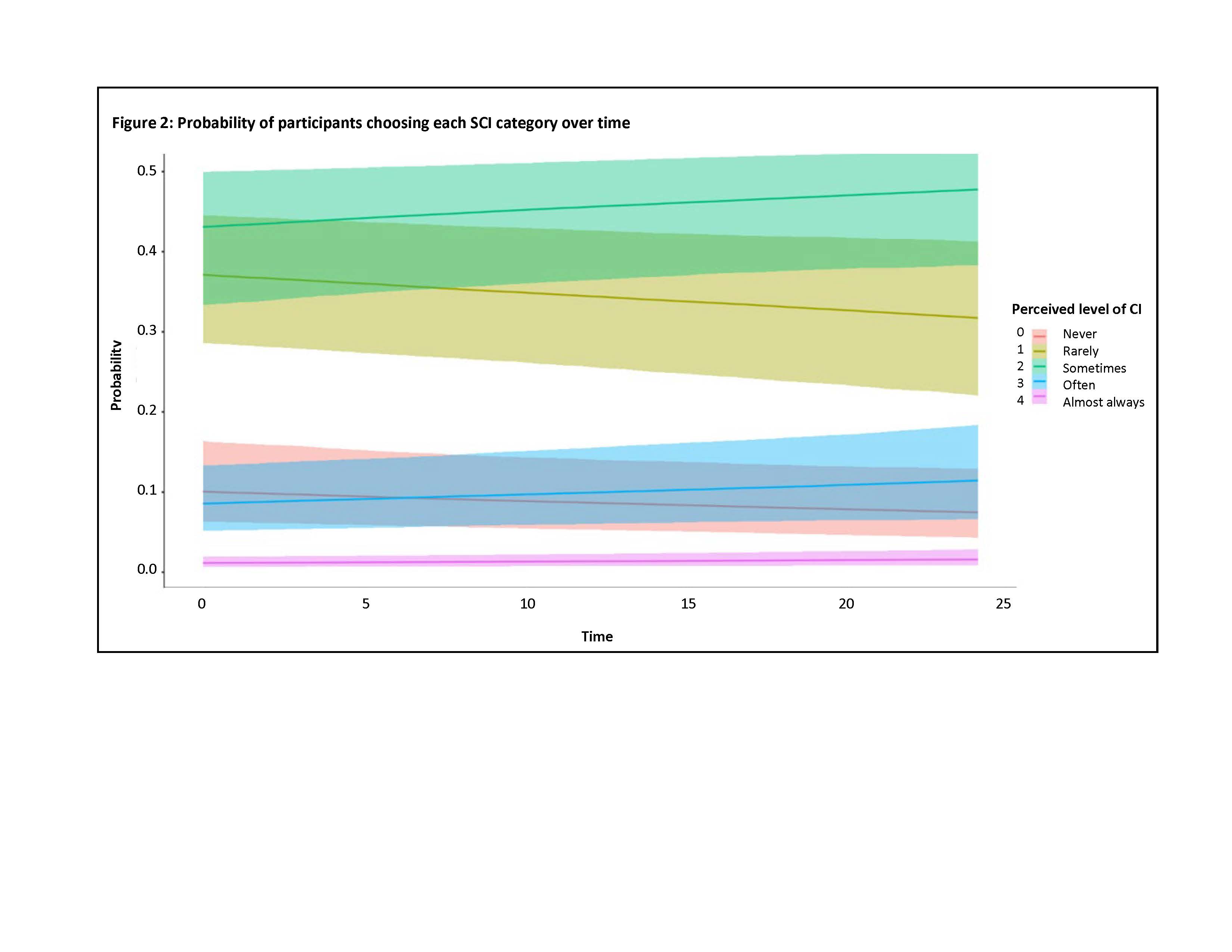 The slopes for the categories, sometimes (2), often (3) and almost always (4) are positive and the slopes for never (0) and rarely (1) are negative. This shows that the probability of participants choosing the more severe categories increases over time whereas selecting the least CI categories decreases.
The slopes for the categories, sometimes (2), often (3) and almost always (4) are positive and the slopes for never (0) and rarely (1) are negative. This shows that the probability of participants choosing the more severe categories increases over time whereas selecting the least CI categories decreases.
Disclosures: M. Barraclough, None; J. Diaz-Martinez, None; A. Knight, None; K. Bingham, None; J. Su, None; M. Kakvan, None; C. Munoz, None; M. Tartaglia, None; L. Ruttan, None; J. Wither, AstraZeneca, Pfizer; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; N. Anderson, None; D. Bonilla, None; S. Appenzeller, None; B. Parker, None; P. Katz, None; D. Beaton, None; R. Green, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); Z. Touma, None.
Background/Purpose: Subjective cognitive impairment (SCI) is a significant problem in SLE and there are a lack of studies assessing change over time in SCI. The Perceived Deficits Questionnaire (PDQ-20) is designed to measure SCI. This tool consists of 20 items assessing different perceived cognitive difficulties. Participants respond using a 5-point Likert scale (scale categories from 0-never to 4-almost always experiencing the difficulty). Using the PDQ-20, the aim of this study was to assess SCI changes over time in an SLE cohort. In addition, the usefulness of each PDQ-20 item as an SCI measure was explored.
Methods: Demographic, clinical, and psychiatric data, patient reported outcomes, including the PDQ-20 and the ACR neuropsychological battery were collected at multiple visits (0, 6, 12 and 24 months) for participants meeting the 2019 EULAR/ACR classification criteria for SLE. Bayesian longitudinal item response theory (IRT) modelling - using time as a fixed effect, and PDQ-20 items and time/participant as random effects - were undertaken to assess SCI changes over time. Model One was fitted with an easiness parameter that was estimated for each PDQ-20 item (higher values represent a higher probability of participants selecting a more severe SCI category). Model Two repeated model one, but also included a discrimination parameter (DP) per item. The DP represented the weight of each PDQ-20 item within the latent trait (SCI). Finally, leave-one-out cross-validation (loo-cv) was used to determine the best statistical model for our data set.
Results: Results from 283 participants for 612 visits were used. Participants were representative of a typical SLE patient group (Table 1). The easiness parameters for each PDQ-20 item showed items 1, 3, 7, 13, and 15 had higher positive values, meaning the chances of selecting one of the more severe SCI categories was increased for these items. Model One showed that the log-odds of SCI increases over time (0.01 95% CrI 0.00-0.03). Model Two indicated that items 1, 14, 17, and 19 held the most discrimination, meaning that these items can distinguish between patients with very similar latent abilities, but the effect of time was no longer significant (0.03 95% CrI -0.11-0.13) (Figure 1). Loo-cv showed model two to be the best fit for our data. Finally, plotting the probability of selecting each PDQ-20 SCI category over time found that the probability of selecting the more severe categories (2-4) increased over time, whereas the less severe (0-1) reduced over time (Figure 2).
Conclusion: This analysis indicated that SCI may increase over time. It also highlighted which items on the PDQ-20 are more likely to be scored with a high SCI and which are more discriminate with regards to the latent trait in our SLE cohort. This complex analysis is the first to have used IRT for the assessment of longitudinal Likert data and did so by fitting the data to one trajectory (worsening SCI over time). Our future analyses will use this new technique to investigate multiple trajectories of SCI over time within our SLE cohort.
 Table 1. Baseline characteristics of the study cohort
Table 1. Baseline characteristics of the study cohort
 The slopes for the categories, sometimes (2), often (3) and almost always (4) are positive and the slopes for never (0) and rarely (1) are negative. This shows that the probability of participants choosing the more severe categories increases over time whereas selecting the least CI categories decreases.
The slopes for the categories, sometimes (2), often (3) and almost always (4) are positive and the slopes for never (0) and rarely (1) are negative. This shows that the probability of participants choosing the more severe categories increases over time whereas selecting the least CI categories decreases.Disclosures: M. Barraclough, None; J. Diaz-Martinez, None; A. Knight, None; K. Bingham, None; J. Su, None; M. Kakvan, None; C. Munoz, None; M. Tartaglia, None; L. Ruttan, None; J. Wither, AstraZeneca, Pfizer; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; N. Anderson, None; D. Bonilla, None; S. Appenzeller, None; B. Parker, None; P. Katz, None; D. Beaton, None; R. Green, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); Z. Touma, None.


