Back
Ignite Talk
Session: Ignite Session 3B
2142: Strong Correlation Between Short- vs Long-form Composite Measures of Psoriatic Arthritis Disease Activity in a TNFi-IR Population Treated with Guselkumab: Data from the Phase 3b COSMOS Trial
Sunday, November 13, 2022
9:35 AM – 9:40 AM Eastern Time
Location: Center City Stage

William Tillett, MD, PhD
Royal National Hospital for rheumatic diseases
Bath, United KingdomDisclosure: Disclosure(s): No relevant disclosure to display
Ignite Speaker(s)
William Tillett1, Laura Coates2, Mohamed Sharaf3, Marlies Neuhold4, Elke Theander5, Paul Bergmans6, May Shawi7, Michelle Perate8, Christine Contré9 and Philip Helliwell10, 1Centre for Therapeutic Innovation, University of Bath, Bath, United Kingdom, 2Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom, 3Johnson & Johnson, Middle East FZ LLC, Dubai, United Arab Emirates, 4Takeda Europe & Canada Business Unit (EUCAN) Medical Affairs, Zurich, Switzerland; formerly at Janssen Scientific Affairs, LLC, Zug, Switzerland, 5Formerly at Janssen Scientific Affairs, LLC, Solna, Sweden, 6Formerly at Janssen, Breda, Netherlands, 7Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 8Janssen Scientific Affairs, LLC, Horsham, PA, 9Formerly at Janssen Scientific Affairs, LLC, Issy-les-Moulineaux, France, Issy-les-Moulineaux, France, 10Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom
Background/Purpose: Accurate assessment of psoriatic arthritis (PsA) disease activity in clinical practice requires a feasible, continuous, multidimensional composite instrument to assess key domains of this heterogeneous disease. While currently available composite tools used in PsA, including the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Composite Exercise (GRACE) and the PsA Disease Activity Score (PASDAS), are useful in clinical trials, their complexity and time required to complete limit their use in clinical practice.1 Abbreviated instruments, known as 3 visual analogue scale (VAS) and 4VAS, have demonstrated good performance in an observational study, but further testing was recommended.2 The purpose of this analysis is to explore the correlation between 3VAS or 4VAS and GRACE, PASDAS and measures of quality of life using data from COSMOS.
Methods: The Phase 3b COSMOS study assessed guselkumab (GUS) 100mg Q8W versus placebo (PBO) in tumor necrosis factor inhibitor (TNFi) inadequate response (IR, inadequate efficacy or intolerance) pts with active PsA.3 Pts who received PBO crossed over to GUS at either Week (W) 16 (early escape [EE], n=45/96) or W24 (planned, n=51/96). Each domain of the 3VAS (physician global, pt global, pt skin) and 4VAS (physician global, pt pain, pt joint, pt skin) was evaluated using a 0–10 VAS (higher score=more active disease), and calculated mean scores were plotted over time in a pt-continuer population (those with W0 and W48 data). Pearson correlation assessed relationships between observed values over time for 3VAS/4VAS versus GRACE, PASDAS, the Health Assessment Questionnaire-Disability Index (HAQ-DI), and the 36-item Short Form Health Survey Physical Component Summary (SF-36 PCS) score.
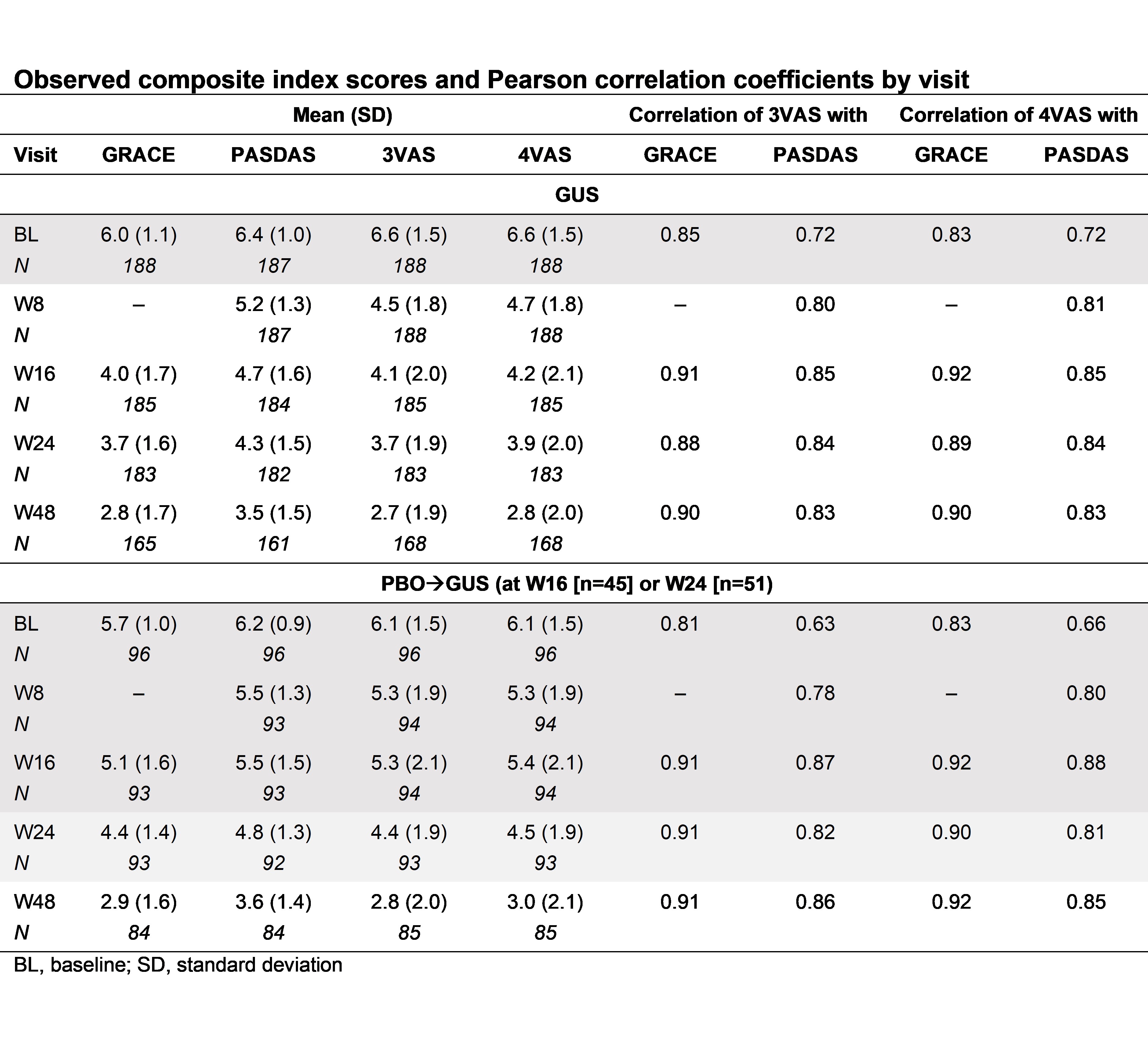
Results: Data from 285 pts were examined (GUS, n=189; PBO, n=96). Substantial improvements ( >45%) were seen in GRACE, PASDAS, 3VAS and 4VAS scores through W48 in GUS‑treated pts (Table), with separation from PBO as early as W8 (Fig). Strong correlations between 3VAS/4VAS and GRACE (r=0.83–0.92)/PASDAS (r=0.72–0.85) were observed in GUS-randomized pts at each visit (Table). 3VAS/4VAS showed moderate correlation with HAQ-DI (r=0.45–0.63) and SF-36 PCS (r=–0.40 to –0.65). Consistent results were observed in PBOàGUS pts.
Conclusion: In these TNFi-IR pts, GUS treatment led to substantial improvements in PsA disease activity through 1 year using several composite indices. All indices, including the 3VAS/4VAS, allowed early discrimination between the GUS and PBO cohorts. The strong correlation between 3VAS/4VAS and GRACE/PASDAS in this pt population is promising and highlights that abbreviated composite indices can provide an accurate assessment of disease activity that may, because of better feasibility, lead to a wider use of such instruments and thus improve pt care. As a next step, thresholds for disease states should be further evaluated.
References
1. Tillett W et al. J Rheumatol Suppl. 2020;96:11–8
2. Tillett W et al. J Rheumatol Suppl. 2021;97:45–9
3. Coates LC et al. Ann Rheum Dis. 2021 (epub)
.jpg)
Disclosures: W. Tillett, Abbvie, Amgen, Eli-Lilly, GlaxoSmithKlein (GSK), Janssen, MSD, Novartis, Pfizer, UCB; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); M. Sharaf, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Neuhold, Janssen Pharmaceutical Companies of Johnson and Johnson, Takeda Pharmaceutical Company; E. Theander, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Bergmans, Janssen Pharmaceutical Companies of Johnson and Johnson, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Perate, Janssen Pharmaceutical Companies of Johnson and Johnson; C. Contré, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis.
Background/Purpose: Accurate assessment of psoriatic arthritis (PsA) disease activity in clinical practice requires a feasible, continuous, multidimensional composite instrument to assess key domains of this heterogeneous disease. While currently available composite tools used in PsA, including the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Composite Exercise (GRACE) and the PsA Disease Activity Score (PASDAS), are useful in clinical trials, their complexity and time required to complete limit their use in clinical practice.1 Abbreviated instruments, known as 3 visual analogue scale (VAS) and 4VAS, have demonstrated good performance in an observational study, but further testing was recommended.2 The purpose of this analysis is to explore the correlation between 3VAS or 4VAS and GRACE, PASDAS and measures of quality of life using data from COSMOS.
Methods: The Phase 3b COSMOS study assessed guselkumab (GUS) 100mg Q8W versus placebo (PBO) in tumor necrosis factor inhibitor (TNFi) inadequate response (IR, inadequate efficacy or intolerance) pts with active PsA.3 Pts who received PBO crossed over to GUS at either Week (W) 16 (early escape [EE], n=45/96) or W24 (planned, n=51/96). Each domain of the 3VAS (physician global, pt global, pt skin) and 4VAS (physician global, pt pain, pt joint, pt skin) was evaluated using a 0–10 VAS (higher score=more active disease), and calculated mean scores were plotted over time in a pt-continuer population (those with W0 and W48 data). Pearson correlation assessed relationships between observed values over time for 3VAS/4VAS versus GRACE, PASDAS, the Health Assessment Questionnaire-Disability Index (HAQ-DI), and the 36-item Short Form Health Survey Physical Component Summary (SF-36 PCS) score.
Results: Data from 285 pts were examined (GUS, n=189; PBO, n=96). Substantial improvements ( >45%) were seen in GRACE, PASDAS, 3VAS and 4VAS scores through W48 in GUS‑treated pts (Table), with separation from PBO as early as W8 (Fig). Strong correlations between 3VAS/4VAS and GRACE (r=0.83–0.92)/PASDAS (r=0.72–0.85) were observed in GUS-randomized pts at each visit (Table). 3VAS/4VAS showed moderate correlation with HAQ-DI (r=0.45–0.63) and SF-36 PCS (r=–0.40 to –0.65). Consistent results were observed in PBOàGUS pts.
Conclusion: In these TNFi-IR pts, GUS treatment led to substantial improvements in PsA disease activity through 1 year using several composite indices. All indices, including the 3VAS/4VAS, allowed early discrimination between the GUS and PBO cohorts. The strong correlation between 3VAS/4VAS and GRACE/PASDAS in this pt population is promising and highlights that abbreviated composite indices can provide an accurate assessment of disease activity that may, because of better feasibility, lead to a wider use of such instruments and thus improve pt care. As a next step, thresholds for disease states should be further evaluated.
References
1. Tillett W et al. J Rheumatol Suppl. 2020;96:11–8
2. Tillett W et al. J Rheumatol Suppl. 2021;97:45–9
3. Coates LC et al. Ann Rheum Dis. 2021 (epub)
.jpg)

Disclosures: W. Tillett, Abbvie, Amgen, Eli-Lilly, GlaxoSmithKlein (GSK), Janssen, MSD, Novartis, Pfizer, UCB; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); M. Sharaf, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Neuhold, Janssen Pharmaceutical Companies of Johnson and Johnson, Takeda Pharmaceutical Company; E. Theander, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Bergmans, Janssen Pharmaceutical Companies of Johnson and Johnson, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Perate, Janssen Pharmaceutical Companies of Johnson and Johnson; C. Contré, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis.

