Back
Ignite Talk
Session: Ignite Session 4A
0439: Clinical Characteristics of Patients with ANCA-Associated Vasculitis with and Without Alpha-1 Antitrypsin Deficiency Alleles
Sunday, November 13, 2022
10:00 AM – 10:05 AM Eastern Time
Location: Northern Liberties Stage
- LF
Lynn Fussner, MD
The Ohio State University
Columbus, OH, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Lynn Fussner1, David Cuthbertson2, Peter Grayson3, David Jayne4, Carol McAlear5, Michael Walsh6, Katherine Siminovitch7, Ulrich Specks8 and Peter Merkel9, 1The Ohio State University, Columbus, OH, 2University of South Florida, Tampa, FL, 3National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH), Bethesda, MD, 4University of Cambridge, Cambridge, United Kingdom, 5Division of Rheumatology, University of Pennsylvania, Philadelphia, PA, USA, Philadelphia, PA, 6McMaster University, Hamilton, ON, Canada, 7University of Toronto, Toronto, ON, Canada, 8Mayo Clinic, Rochester, MN, 9University of Pennsylvania, Philadelphia, PA
Background/Purpose: There are multiple reasons to suspect that alpha-1 antitrypsin (A1AT) genotype impacts disease characteristics in ANCA-associated vasculitis (AAV). A1AT serves as the predominant endogenous inhibitor of proteinase 3 (PR3), a major target antigen of ANCA, and interferes with ANCA-induced activation of both monocytes and neutrophils. Additionally, two separate, large genome-wide association studies found that mutations in SERPINA1, the gene encoding A1AT, are associated with increased risk of developing AAV. This report describes the clinical characteristics of a large cohort of patients with AAV with and without A1AT deficiency alleles.
Methods: DNA was obtained from patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), two forms of AAV, and linked to clinical data utilizing standardized forms. Demographics and clinical characteristics are described and compared between patients with and without A1AT deficiency alleles. Disease manifestations were categorized as representative of capillaritis or granulomatous inflammation, or neither (as with constitutional symptoms, for example).
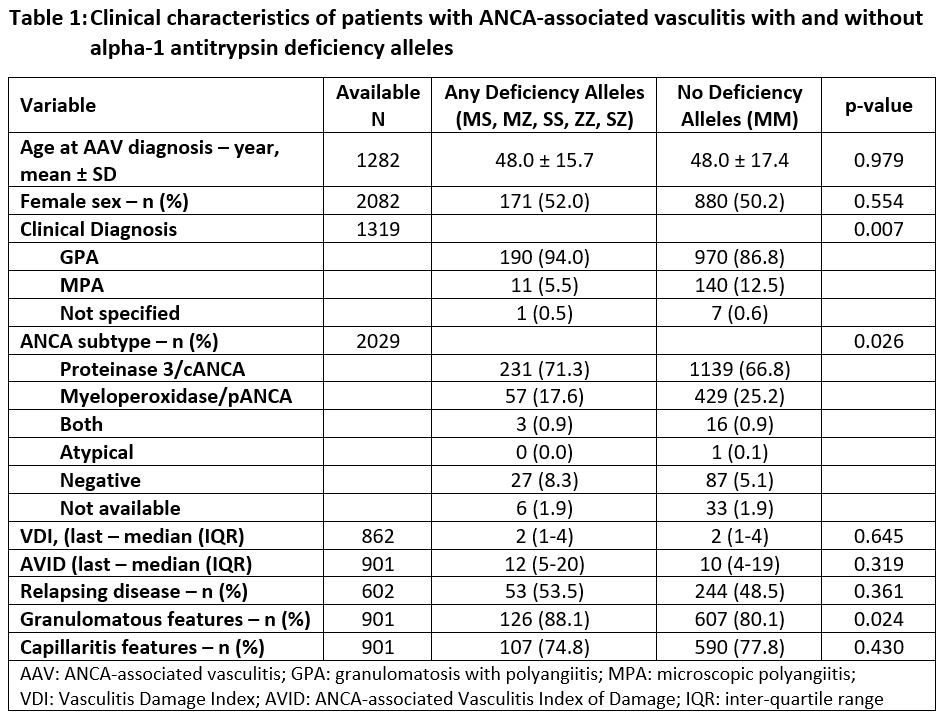
Results: Genotype data from 2145 unique patients with GPA and MPA were identified and analyzed. While 1805 (84.2%) had normal A1AT genotype (MM), 340 patients (15.9%) had at least one A1AT deficiency allele (Z and/or S) – 203 (9.5%) with any S alleles, 142 (6.6%) with any Z alleles. Among these, MS was the most frequently identified abnormal genotype with 186 patients (8.7%), followed by MZ (127, 5.9%), SS (12, 0.6%), ZZ (10, 0.5%), and SZ (5, 0.2%). Clinical characteristics of patients with and without A1AT deficiency alleles are presented in Table 1. Mean age at AAV diagnosis was similar among patients with (48.3 ± 15.9 years) and without A1AT deficiency alleles (48.4 ± 17.8 years among those with MM, p=0.950), as was sex (51.0% female among those with deficiency alleles vs. 49.2% among MM, p=0.533). Patients with deficiency alleles were more frequently described as having a clinical phenotype of GPA (90.3% with deficiency alleles vs. 83.5% MM, p=0.003), and were more frequently PR3-ANCA positive (74.5% with deficiency alleles vs. 63.3% MM, p< 0.001). Additionally, more patients with deficiency alleles were ANCA-negative (5.2% with deficiency alleles vs. 2.9% MM, p=0.029). Patients with deficiency alleles were more likely overall to have granulomatous manifestations of AAV (88.1% with deficiency alleles vs. 80.1% MM, p=0.024), while there was no difference in the frequency of capillaritis manifestations (74.8% with deficiency alleles vs. 77.8% MM, p=0.430).
Conclusion: In this large cohort of patients with AAV nearly 1/6 had one or more A1AT deficiency alleles. MS, a genotype that is often not associated with clinically significant decreases in A1AT levels, was the most frequently identified abnormal genotype among these patients with AAV. The presence of A1AT deficiency alleles was associated with clinical phenotype and ANCA serotype. Further investigation is indicated to evaluate the impact of individual A1AT deficiency alleles on clinical manifestations in AAV.
Disclosures: L. Fussner, None; D. Cuthbertson, None; P. Grayson, None; D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; C. McAlear, None; M. Walsh, Bayer, Vifor, Novo Nordisc, Otsuka; K. Siminovitch, Regeneron; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.
Background/Purpose: There are multiple reasons to suspect that alpha-1 antitrypsin (A1AT) genotype impacts disease characteristics in ANCA-associated vasculitis (AAV). A1AT serves as the predominant endogenous inhibitor of proteinase 3 (PR3), a major target antigen of ANCA, and interferes with ANCA-induced activation of both monocytes and neutrophils. Additionally, two separate, large genome-wide association studies found that mutations in SERPINA1, the gene encoding A1AT, are associated with increased risk of developing AAV. This report describes the clinical characteristics of a large cohort of patients with AAV with and without A1AT deficiency alleles.
Methods: DNA was obtained from patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), two forms of AAV, and linked to clinical data utilizing standardized forms. Demographics and clinical characteristics are described and compared between patients with and without A1AT deficiency alleles. Disease manifestations were categorized as representative of capillaritis or granulomatous inflammation, or neither (as with constitutional symptoms, for example).
Results: Genotype data from 2145 unique patients with GPA and MPA were identified and analyzed. While 1805 (84.2%) had normal A1AT genotype (MM), 340 patients (15.9%) had at least one A1AT deficiency allele (Z and/or S) – 203 (9.5%) with any S alleles, 142 (6.6%) with any Z alleles. Among these, MS was the most frequently identified abnormal genotype with 186 patients (8.7%), followed by MZ (127, 5.9%), SS (12, 0.6%), ZZ (10, 0.5%), and SZ (5, 0.2%). Clinical characteristics of patients with and without A1AT deficiency alleles are presented in Table 1. Mean age at AAV diagnosis was similar among patients with (48.3 ± 15.9 years) and without A1AT deficiency alleles (48.4 ± 17.8 years among those with MM, p=0.950), as was sex (51.0% female among those with deficiency alleles vs. 49.2% among MM, p=0.533). Patients with deficiency alleles were more frequently described as having a clinical phenotype of GPA (90.3% with deficiency alleles vs. 83.5% MM, p=0.003), and were more frequently PR3-ANCA positive (74.5% with deficiency alleles vs. 63.3% MM, p< 0.001). Additionally, more patients with deficiency alleles were ANCA-negative (5.2% with deficiency alleles vs. 2.9% MM, p=0.029). Patients with deficiency alleles were more likely overall to have granulomatous manifestations of AAV (88.1% with deficiency alleles vs. 80.1% MM, p=0.024), while there was no difference in the frequency of capillaritis manifestations (74.8% with deficiency alleles vs. 77.8% MM, p=0.430).
Conclusion: In this large cohort of patients with AAV nearly 1/6 had one or more A1AT deficiency alleles. MS, a genotype that is often not associated with clinically significant decreases in A1AT levels, was the most frequently identified abnormal genotype among these patients with AAV. The presence of A1AT deficiency alleles was associated with clinical phenotype and ANCA serotype. Further investigation is indicated to evaluate the impact of individual A1AT deficiency alleles on clinical manifestations in AAV.

Disclosures: L. Fussner, None; D. Cuthbertson, None; P. Grayson, None; D. Jayne, Aurinia, AstraZeneca, GlaxoSmithKline (GSK), Roche/Genentech, Vifor, Bristol-Myers Squibb(BMS), Chemocentryx, Novartis, Takeda, Boehringer-Ingelheim, Otsuka, UCB, Amgen, Kessai; C. McAlear, None; M. Walsh, Bayer, Vifor, Novo Nordisc, Otsuka; K. Siminovitch, Regeneron; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.

