Back
Ignite Talk
Session: Ignite Session 4B
1809: Impact of Colchicine Prophylaxis on Cardiovascular Outcome Among Gout Patients: A Secondary Analysis of CARES Trial
Sunday, November 13, 2022
10:10 AM – 10:15 AM Eastern Time
Location: Center City Stage
- KH
Keigo Hayashi, MPH
Brigham and Women's Hospital
Brookline, MA, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Keigo Hayashi1, yuqing zhang2, Hyon Choi3 and Kazuki Yoshida4, 1Brigham and Women's Hospital, Okayama, Japan, 2Massachusetts General Hospital, Quincy, MA, 3MASSACHUSETTS GENERAL HOSPITAL, Lexington, MA, 4Brigham and Women's Hospital, Boston, MA
Background/Purpose: Low-dose colchicine has been demonstrated to reduce cardiovascular (CV) events in two recent large cardiovascular trials. Gout patients, for whom colchicine is a highly relevant medication, were unfortunately underrepresented (8% or less) in these CV trials. We aimed to examine the potential CV benefit of colchicine gout flare prophylaxis among established gout patients.
Methods: We conducted an observational re-analysis of the CARES trial. CARES enrolled 6,190 established gout patients with a history of major cardiovascular disease, randomized them for urate-lowering therapy with allopurinol or febuxostat and followed them for a median of 32 months (maximum, 85 months). In addition to the urate-lowering agents, colchicine or non-steroidal anti-inflammatory drugs (NSAIDs) were administered at the investigators’ discretion for gout flare prophylaxis. We first described demographic and cardiovascular factors as baseline covariates and their balance among the three prophylaxis groups: colchicine, NSAIDs and no prophylaxis. We then conducted three group propensity score weighting via matching weights to control for the baseline confounding and confirmed improved covariate balance. Cumulative incidence curves for MACE (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or unstable angina with urgent revascularization) were created before and after propensity score weighting. Cox proportional-hazard models were used to analyze the time to the first occurrence of MACE events.
Results: We identified 6177 patients with reported prophylaxis in the CARES trial: mean (SD) age 64.8 (8.5) years, mean (SD) gout duration 11.8 (11.3) years. Among them, 5,195 patients received colchicine prophylaxis, 662 patients received NSAIDs, and 320 patients were not given gout flare prophylaxis. The baseline characteristics (Table 1) showed that colchicine prophylaxis patients were older, had a higher serum urate level, BMI, had a higher proportion of men and were more likely to have CV comorbidities compared to NSAIDs or no prophylaxis patients. After weighting via matching weights, these characteristics were well balanced. In the unweighted cox proportional-hazard model, the hazard of MACE in the colchicine group was higher than in the NSAIDs group (hazard ratio[HR] 1.44 [95% CI, 1.08 to 1.92]) and similar to the no prophylaxis group (HR 1.30 [95% CI, 0.87 to 1.95]) (Figure1). In the weighted model, however, there was no statistically significant difference in the hazard of MACE events between the three groups (colchicine vs. NSAIDs, HR 1.16 [95% CI, 0.86 to 1.42]; colchicine vs. no prophylaxis, HR 1.32 [95% CI, 0.88 to 1.99). The median (IQR) duration of administration of prophylaxis was 184 (180-195) days in the colchicine group and 182 (118-192) days in the NSAIDs group (Figure2).
Conclusion: We could not find a CV benefit of colchicine gout flare prophylaxis among established gout patients in the CARES trial. However, the typical duration of prophylaxis was 6 months, shorter than major CV trials with colchicine. Further study with longer duration of prophylaxis might be needed to examine the beneficial effect of colchicine among gout patients initiating urate-lowering therapy.
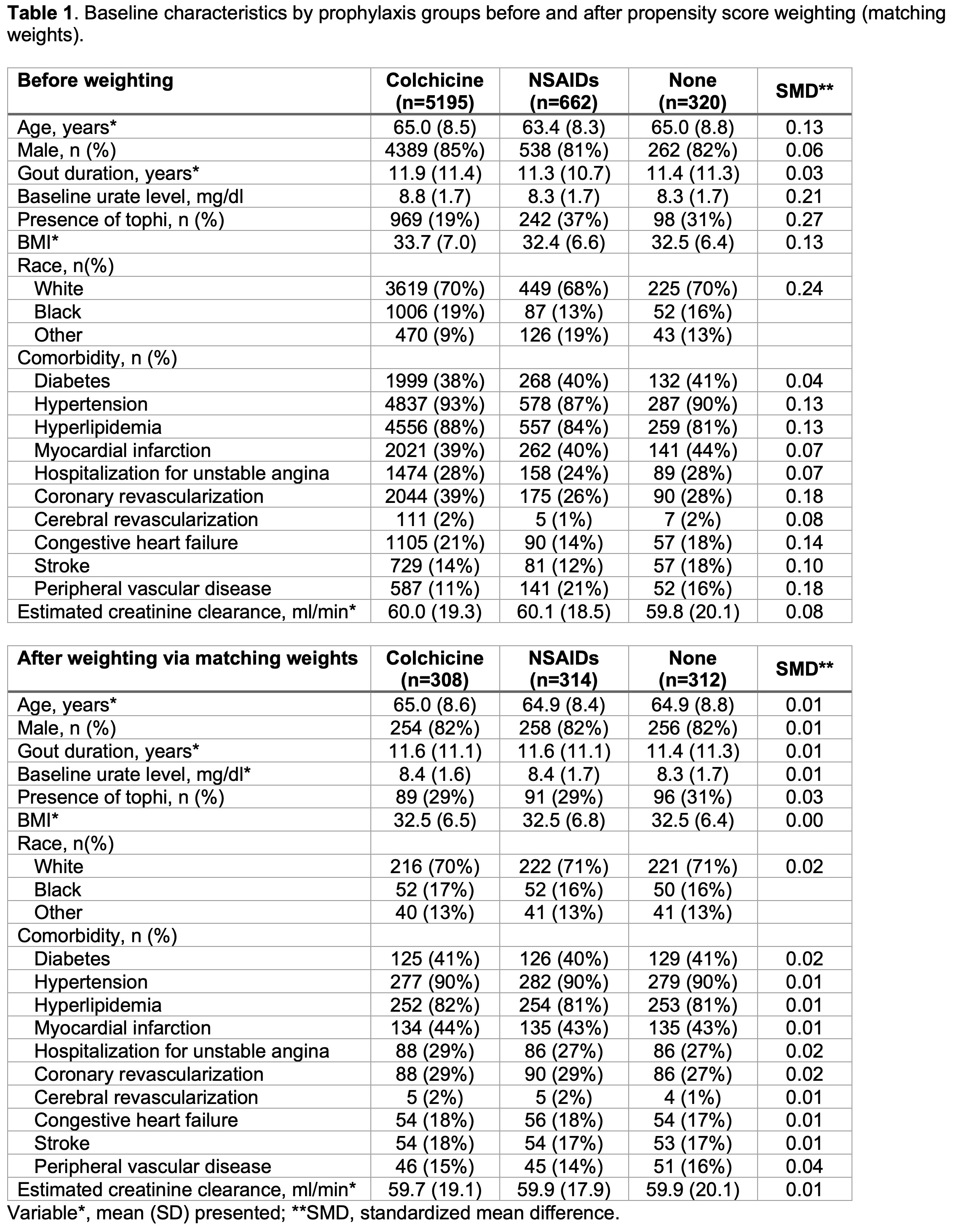 Table 1. Baseline characteristics by prophylaxis groups before and after propensity score weighting (matching
Table 1. Baseline characteristics by prophylaxis groups before and after propensity score weighting (matching
weights).
.jpg) Figure 1. Major adverse cardiovascular event incidence before and after propensity score weighting (matching weights).
Figure 1. Major adverse cardiovascular event incidence before and after propensity score weighting (matching weights).
.jpg) Figure 2. Typical prophylaxis duration.
Figure 2. Typical prophylaxis duration.
Disclosures: K. Hayashi, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix; K. Yoshida, None.
Background/Purpose: Low-dose colchicine has been demonstrated to reduce cardiovascular (CV) events in two recent large cardiovascular trials. Gout patients, for whom colchicine is a highly relevant medication, were unfortunately underrepresented (8% or less) in these CV trials. We aimed to examine the potential CV benefit of colchicine gout flare prophylaxis among established gout patients.
Methods: We conducted an observational re-analysis of the CARES trial. CARES enrolled 6,190 established gout patients with a history of major cardiovascular disease, randomized them for urate-lowering therapy with allopurinol or febuxostat and followed them for a median of 32 months (maximum, 85 months). In addition to the urate-lowering agents, colchicine or non-steroidal anti-inflammatory drugs (NSAIDs) were administered at the investigators’ discretion for gout flare prophylaxis. We first described demographic and cardiovascular factors as baseline covariates and their balance among the three prophylaxis groups: colchicine, NSAIDs and no prophylaxis. We then conducted three group propensity score weighting via matching weights to control for the baseline confounding and confirmed improved covariate balance. Cumulative incidence curves for MACE (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or unstable angina with urgent revascularization) were created before and after propensity score weighting. Cox proportional-hazard models were used to analyze the time to the first occurrence of MACE events.
Results: We identified 6177 patients with reported prophylaxis in the CARES trial: mean (SD) age 64.8 (8.5) years, mean (SD) gout duration 11.8 (11.3) years. Among them, 5,195 patients received colchicine prophylaxis, 662 patients received NSAIDs, and 320 patients were not given gout flare prophylaxis. The baseline characteristics (Table 1) showed that colchicine prophylaxis patients were older, had a higher serum urate level, BMI, had a higher proportion of men and were more likely to have CV comorbidities compared to NSAIDs or no prophylaxis patients. After weighting via matching weights, these characteristics were well balanced. In the unweighted cox proportional-hazard model, the hazard of MACE in the colchicine group was higher than in the NSAIDs group (hazard ratio[HR] 1.44 [95% CI, 1.08 to 1.92]) and similar to the no prophylaxis group (HR 1.30 [95% CI, 0.87 to 1.95]) (Figure1). In the weighted model, however, there was no statistically significant difference in the hazard of MACE events between the three groups (colchicine vs. NSAIDs, HR 1.16 [95% CI, 0.86 to 1.42]; colchicine vs. no prophylaxis, HR 1.32 [95% CI, 0.88 to 1.99). The median (IQR) duration of administration of prophylaxis was 184 (180-195) days in the colchicine group and 182 (118-192) days in the NSAIDs group (Figure2).
Conclusion: We could not find a CV benefit of colchicine gout flare prophylaxis among established gout patients in the CARES trial. However, the typical duration of prophylaxis was 6 months, shorter than major CV trials with colchicine. Further study with longer duration of prophylaxis might be needed to examine the beneficial effect of colchicine among gout patients initiating urate-lowering therapy.
 Table 1. Baseline characteristics by prophylaxis groups before and after propensity score weighting (matching
Table 1. Baseline characteristics by prophylaxis groups before and after propensity score weighting (matchingweights).
.jpg) Figure 1. Major adverse cardiovascular event incidence before and after propensity score weighting (matching weights).
Figure 1. Major adverse cardiovascular event incidence before and after propensity score weighting (matching weights)..jpg) Figure 2. Typical prophylaxis duration.
Figure 2. Typical prophylaxis duration.Disclosures: K. Hayashi, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix; K. Yoshida, None.

