Back
Ignite Talk
Session: Ignite Session 4B
1829: Efficacy and Safety of Allopurinol and Febuxostat in Patients with Gout and Chronic Kidney Disease: A Subgroup Analysis of the STOP Gout Study
Sunday, November 13, 2022
10:20 AM – 10:25 AM Eastern Time
Location: Center City Stage
- LH
Lindsay Helget, MD
University of Nebraska Medical Center
Omaha, NE, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Lindsay Helget1, Anne Davis-Karim2, James O'Dell1, Ted Mikuls3, Jeff Newcomb1, Maria Androsenko4, Mary Brophy4, Bryant England1, Ryan Ferguson4, Michael Pillinger5, Tuhina Neogi6, Hongsheng Wu4 and Paul Palevsky7, 1University of Nebraska Medical Center, Omaha, NE, 2VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM, 3Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE, 4VA Boston Cooperative Studies Program Coordinating Center, Boston, MA, 5NYU Grossman School of Medicine, New York, NY, 6Boston University School of Medicine, Boston, MA, 7University of Pittsburgh School of Medicine, Pittsburgh, Pittsburgh
Background/Purpose: Urate lowering therapy (ULT) is a cornerstone in the treatment of gout, which afflicts over 2 million individuals with chronic kidney disease (CKD) in the US. Limited data exist on the comparative effectiveness of allopurinol and febuxostat, the two most common ULTs, in patients with CKD. Using data from the recently completed multicenter, randomized, double-blind STOP Gout trial comparing the efficacy and safety of allopurinol and febuxostat in the management of gout (O’Dell JR et al. NEJM Evidence 2022), we evaluated the efficacy and safety of these medications in the subgroup of patients with CKD.
Methods: Patients with gout and serum urate (SU) concentration ≥6.8 mg/dL were randomized 1:1 to receive allopurinol or febuxostat. The trial protocol specified that at least one-third of participants would have CKD stage III, defined by an eGFR 30-59 mL/min/1.73 m2 at enrollment, and patients with an eGFR < 30 mL/min/1.73 m2 were excluded. ULT was titrated during weeks 0-24 (Phase 1) and maintained during weeks 25-48 (Phase 2), with escalation as necessary, to reach goal SU of < 6.0 mg/dL. Participants were observed on a stable ULT dose during weeks 49-72 (Phase 3). Primary outcome was the occurrence of ≥1 gout flare during Phase 3. Secondary outcomes included achievement of SU < 6 mg/dL at end of Phase 2 and serious adverse events (SAEs). Outcomes in this prespecified sub-analysis were compared between treatment groups using two-sided chi square tests or Students t-tests.
Results: Of the 940 trial participants, 351 (37.3%) had CKD; 277 (29.5%) had flare data from Phase 3. Phase 3 flare data was missing for 31 febuxostat and 43 allopurinol patients. Among those with CKD, baseline characteristics were similar by treatment group, except cardiovascular disease frequency was more common in those randomized to allopurinol and BMI was higher in those given febuxostat (Table 1). Fewer participants randomized to allopurinol had ≥1 gout flare during phase 3 (32% v 45%; p=0.02) despite a similar proportion achieving a SU < 6.0 mg/dL in Phase 2 (Table 2). There were no treatment-arm differences in overall SAEs, study withdrawal due to SAE, or in the proportion with cardiovascular events. More participants had rash with allopurinol (1 severe, the rest mild-to-moderate). Fifteen participants with CKD on allopurinol experienced acute kidney injury (AKI) during the trial vs. 4 on febuxostat; all but one CKD patient who experienced AKI was on diuretic therapy at trial initiation. Of the 19 CKD participants who experienced AKI across both treatment arms, 79% had complete recovery of renal function.
Conclusion: This sub-analysis from a large, randomized double-blind, non-inferiority trial demonstrated that allopurinol and febuxostat were similarly efficacious in achieving target SU goals. Although loss to follow-up was quantitatively higher with allopurinol, study withdrawal related to safety issues was similar by treatment arm. Further study will be needed to confirm whether the signal for increased AKI observed with allopurinol treatment is replicated in larger populations and, if confirmed, to better understand mechanisms underpinning this observation.
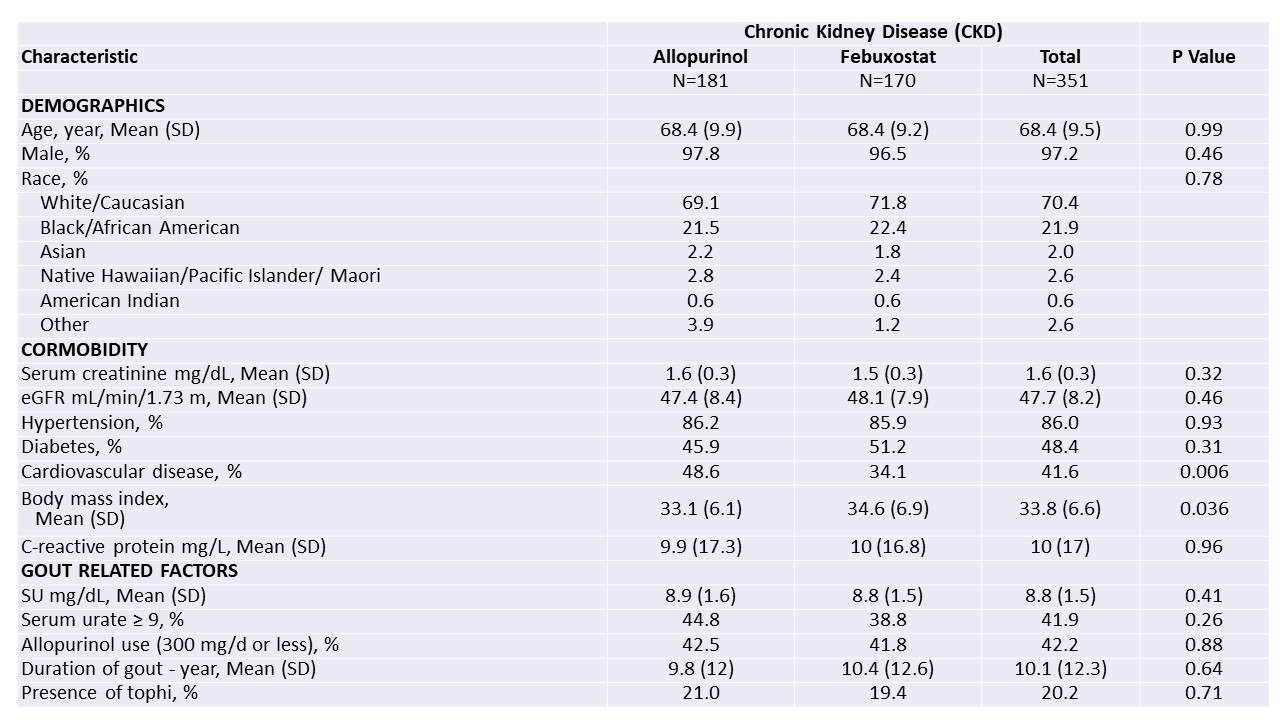 Table 1. Baseline characteristics of trial participants with CKD
Table 1. Baseline characteristics of trial participants with CKD
*Values are presented as percentages or means (SD); eGFR denotes estimated glomerular filtration rate; hypertension and diabetes were documented for study using participants’ problem lists in medical record review; cardiovascular disease indicates a history of coronary artery disease, myocardial infarction, or heart failure; body mass index is the weight in kilograms divided by the square of the height in meters
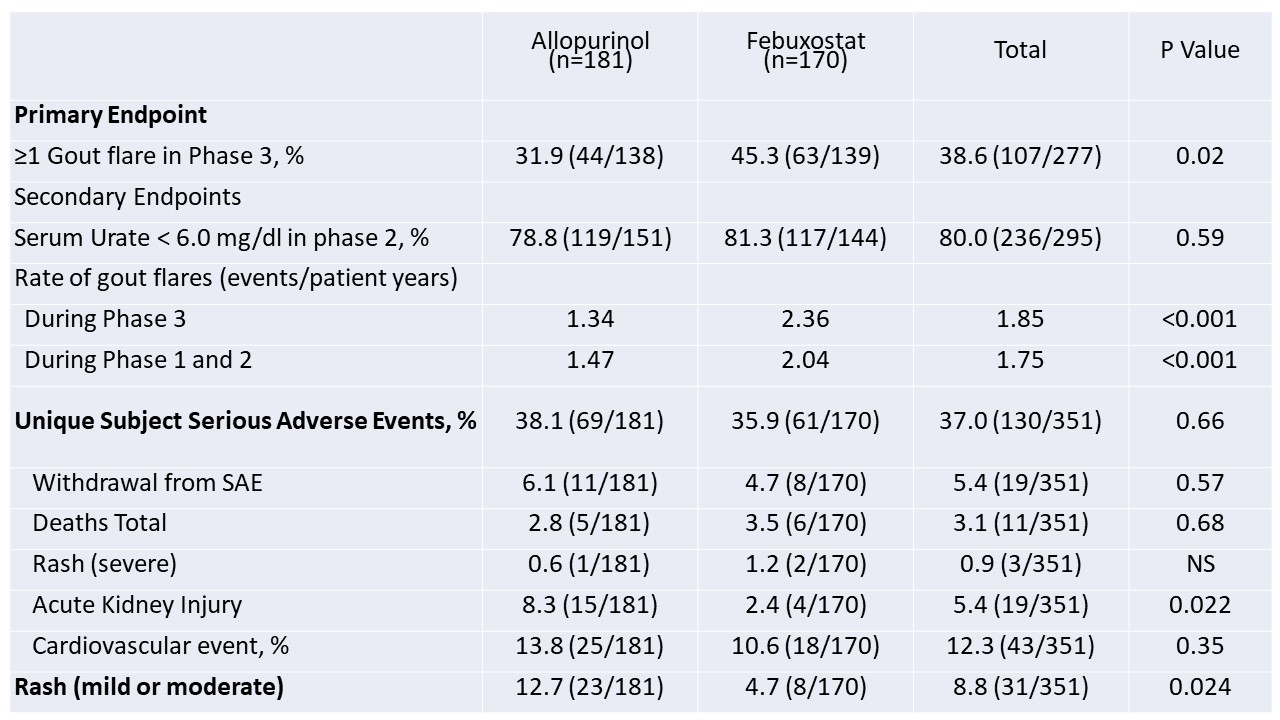 Table 2. Results of primary and secondary end points in trial participants with CKD
Table 2. Results of primary and secondary end points in trial participants with CKD
Disclosures: L. Helget, None; A. Davis-Karim, None; J. O'Dell, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; J. Newcomb, None; M. Androsenko, None; M. Brophy, None; B. England, Boehringer-Ingelheim; R. Ferguson, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; H. Wu, None; P. Palevsky, None.
Background/Purpose: Urate lowering therapy (ULT) is a cornerstone in the treatment of gout, which afflicts over 2 million individuals with chronic kidney disease (CKD) in the US. Limited data exist on the comparative effectiveness of allopurinol and febuxostat, the two most common ULTs, in patients with CKD. Using data from the recently completed multicenter, randomized, double-blind STOP Gout trial comparing the efficacy and safety of allopurinol and febuxostat in the management of gout (O’Dell JR et al. NEJM Evidence 2022), we evaluated the efficacy and safety of these medications in the subgroup of patients with CKD.
Methods: Patients with gout and serum urate (SU) concentration ≥6.8 mg/dL were randomized 1:1 to receive allopurinol or febuxostat. The trial protocol specified that at least one-third of participants would have CKD stage III, defined by an eGFR 30-59 mL/min/1.73 m2 at enrollment, and patients with an eGFR < 30 mL/min/1.73 m2 were excluded. ULT was titrated during weeks 0-24 (Phase 1) and maintained during weeks 25-48 (Phase 2), with escalation as necessary, to reach goal SU of < 6.0 mg/dL. Participants were observed on a stable ULT dose during weeks 49-72 (Phase 3). Primary outcome was the occurrence of ≥1 gout flare during Phase 3. Secondary outcomes included achievement of SU < 6 mg/dL at end of Phase 2 and serious adverse events (SAEs). Outcomes in this prespecified sub-analysis were compared between treatment groups using two-sided chi square tests or Students t-tests.
Results: Of the 940 trial participants, 351 (37.3%) had CKD; 277 (29.5%) had flare data from Phase 3. Phase 3 flare data was missing for 31 febuxostat and 43 allopurinol patients. Among those with CKD, baseline characteristics were similar by treatment group, except cardiovascular disease frequency was more common in those randomized to allopurinol and BMI was higher in those given febuxostat (Table 1). Fewer participants randomized to allopurinol had ≥1 gout flare during phase 3 (32% v 45%; p=0.02) despite a similar proportion achieving a SU < 6.0 mg/dL in Phase 2 (Table 2). There were no treatment-arm differences in overall SAEs, study withdrawal due to SAE, or in the proportion with cardiovascular events. More participants had rash with allopurinol (1 severe, the rest mild-to-moderate). Fifteen participants with CKD on allopurinol experienced acute kidney injury (AKI) during the trial vs. 4 on febuxostat; all but one CKD patient who experienced AKI was on diuretic therapy at trial initiation. Of the 19 CKD participants who experienced AKI across both treatment arms, 79% had complete recovery of renal function.
Conclusion: This sub-analysis from a large, randomized double-blind, non-inferiority trial demonstrated that allopurinol and febuxostat were similarly efficacious in achieving target SU goals. Although loss to follow-up was quantitatively higher with allopurinol, study withdrawal related to safety issues was similar by treatment arm. Further study will be needed to confirm whether the signal for increased AKI observed with allopurinol treatment is replicated in larger populations and, if confirmed, to better understand mechanisms underpinning this observation.
 Table 1. Baseline characteristics of trial participants with CKD
Table 1. Baseline characteristics of trial participants with CKD*Values are presented as percentages or means (SD); eGFR denotes estimated glomerular filtration rate; hypertension and diabetes were documented for study using participants’ problem lists in medical record review; cardiovascular disease indicates a history of coronary artery disease, myocardial infarction, or heart failure; body mass index is the weight in kilograms divided by the square of the height in meters
 Table 2. Results of primary and secondary end points in trial participants with CKD
Table 2. Results of primary and secondary end points in trial participants with CKDDisclosures: L. Helget, None; A. Davis-Karim, None; J. O'Dell, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; J. Newcomb, None; M. Androsenko, None; M. Brophy, None; B. England, Boehringer-Ingelheim; R. Ferguson, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; H. Wu, None; P. Palevsky, None.

