Back
Abstract Session
Session: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases II (1106–1111)
1106: Effect of Tocilizumab on Disease Activity in Patients with Active Polymyalgia Rheumatica on Glucocorticoid Therapy: A Randomized Clinical Trial
Sunday, November 13, 2022
10:30 AM – 10:40 AM Eastern Time
Location: Room 119
- VD
Valerie Devauchelle, MD, PhD
UBO
Brest, France
Presenting Author(s)
Valerie Devauchelle1, Guillermo CARVAJAL ALEGRIA2, Emmanuelle Dernis3, Christophe Richez4, Marie Truchetet5, Daniel Wendling6, ERIC TOUSSIROT7, aleth Perdriger8, jacques-eric gottenberg9, Renaud FELTEN10, Bruno Fautrel11, laurent chiche12, Pascal HILLIQUIN13, Catherine Le Henaff14, Benjamin Dervieux15, Guillaume Direz16, Isabelle Chary-Valckenaere17, Divi CORNEC18, Dewi Guellec19, Thierry MARHADOUR20, Emmanule Nowak19 and Alain Saraux21, 1Université de Bretagne Occidentale, Brest, France, 2CHRU de Tours, Tours, France, 3LE MANS general hospital, LE MANS, France, 4Université de Bordeaux, Bordeaux, France, 5Bordeaux University Hospital, Bordeaux, France, 6CHU, University Teaching Hospital, Besançon, France, 7CHU de Besançon, Besançon, France, 8Rennes University, Rennes, France, 9Strasbourg University Hospital, Strasbourg, France, 10Hôpitaux Universitaires de Strasbourg, Strasbourg, France, 11Sorbonne University Paris, France and Pierre Louis Institute of Epidemiology and Public Health, Paris, France, Paris, France, 12hopital europeen, Marseille, France, 13Centre Hospitalier Sud-Francilien, Corbeil-Essonnes, France, 14Morlaix Hospital, Morlaix, France, 15Mulhouse Hospital, Mulhouse, France, 16Le Mans Hospital, Le Mans, France, 17Nancy University Hospital, Vandoeuvre, France, 18CHRU Brest, Brest, France, 19Brest University Hospital, Brest, France, 20CHU Cavale Blanche, Brest, France, 21CHU Brest, Brest, France
Background/Purpose: Few treatments are available for patients with glucocorticoid-dependent polymyalgia rheumatica (PMR). Interleukin-6 antagonists deserve evaluation in active glucocorticoid-dependent PMR.
Our objective was to compare the efficacy of tocilizumab vs. placebo in patients with glucocorticoid-dependent polymyalgia rheumatica.
Methods: Design, Setting, and Participants. This double-blind parallel-group placebo-controlled randomized clinical trial enrolled 101 patients at 17 hospitals in France from February 2017 to October 2019; last follow-up was in November 2020. Inclusion criteria were persistent disease activity (CRP PMR-AS >10) and prednisone ≥10 mg/day. Tocilizumab was donated by Roche and by Chugai.
Interventions. Patients were randomly assigned to intravenous tocilizumab (8 mg/Kg) (n=51) or placebo (n=50) every 4 weeks for 24 weeks, combined with predefined standardized oral prednisone tapering.
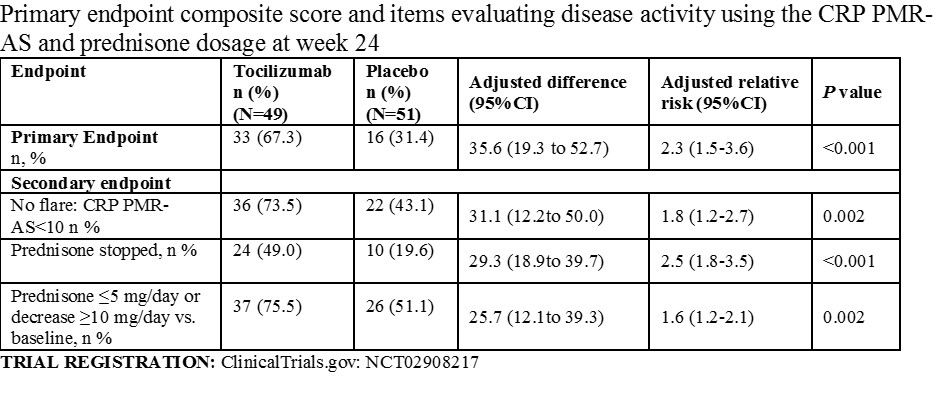
Main Outcomes and Measures. The primary efficacy endpoint was CRP PMR-AS< 10 (Min=0-Max 100 with higher values indicating greater activity, no MCID defined) combined with either prednisone ≤5 mg/day or a ≥10-mg prednisone decrease at week 24. There were 11 secondary outcomes assessed at week 24 included in this report, including disease activity and proportion of patients who were no longer taking prednisone.
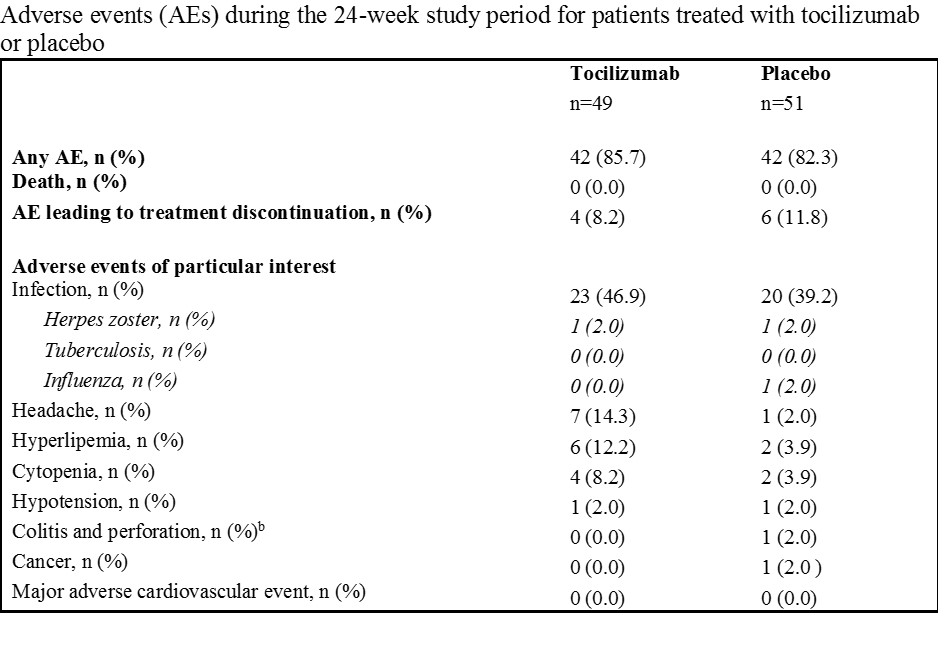
Results: Of the 101 patients randomized (mean age, 67.2 years; 68 [67.3%] females), 100 (99%) received at least one infusion and 100% completed the trial. The primary endpoint was achieved in 67.3% and 31.4% of patients in the tocilizumab and placebo groups, respectively ( adjusted relative risk, 2.3; 95% confidence interval [95%CI]: 1.5 to 3.6; P< 0.001). Of 11 reported secondary endpoints at week 24, seven showed significant differences favoring tocilizumab including mean CRP PMR-AS score (7.5 [95% CI, 5.4 to 9.6] vs. 14.9 [95% CI, 11.4 to 18.4]; adjusted difference, 7.5 with 95%CI 3.8 to 11.2; P< 0.001) and proportion of prednisone-free patients (49.0% vs. 19.6%; adjusted relative risk, 2.5; 95%CI: 1.8 to 3.5; P< 0.001). The most frequent adverse events were infections, experienced by 23 (46.9%) patients in the tocilizumab group and 20 (39.2%) in the placebo group.
Conclusion: Among patients with active polymyalgia rheumatica despite prednisone therapy, tocilizumab, compared to a placebo, resulted in a significantly greater proportion of patients achieving a CPR PMR-AS< 10 with reduced prednisone requirements at week 24. However, additional trials are needed to confirm efficacy and to determine the balance of potential benefits and harms.
Disclosures: V. Devauchelle, Pfizer, Novartis, AbbVie/Abbott, Novartis, Bristol-Myers Squibb(BMS), Roche-Chugai, Galapados; G. CARVAJAL ALEGRIA, Roche-Chugai; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; M. Truchetet, AbbVie/Abbott, Pfizer, Eli Lilly, Galapados; D. Wendling, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Pfizer, Roche, Amgen, Nordic Pharma, UCB, Novartis, Janssen, Eli Lilly, Sandoz, Galapados, Grunenthal; E. TOUSSIROT, None; a. Perdriger, None; j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; R. FELTEN, Novartis, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Nordic Pharma, Merck/MSD, MEDAC, Pfizer, Sanofi, UCB; B. Fautrel, Pfizer, Novartis, Roche, Sanofi-Aventis, SOBI, UCB; l. chiche, Novartis, Bristol-Myers Squibb(BMS); P. HILLIQUIN, None; C. Le Henaff, None; B. Dervieux, None; G. Direz, Novartis, Roche, Sanofi; I. Chary-Valckenaere, None; D. CORNEC, None; D. Guellec, None; T. MARHADOUR, None; E. Nowak, None; A. Saraux, None.
Background/Purpose: Few treatments are available for patients with glucocorticoid-dependent polymyalgia rheumatica (PMR). Interleukin-6 antagonists deserve evaluation in active glucocorticoid-dependent PMR.
Our objective was to compare the efficacy of tocilizumab vs. placebo in patients with glucocorticoid-dependent polymyalgia rheumatica.
Methods: Design, Setting, and Participants. This double-blind parallel-group placebo-controlled randomized clinical trial enrolled 101 patients at 17 hospitals in France from February 2017 to October 2019; last follow-up was in November 2020. Inclusion criteria were persistent disease activity (CRP PMR-AS >10) and prednisone ≥10 mg/day. Tocilizumab was donated by Roche and by Chugai.
Interventions. Patients were randomly assigned to intravenous tocilizumab (8 mg/Kg) (n=51) or placebo (n=50) every 4 weeks for 24 weeks, combined with predefined standardized oral prednisone tapering.
Main Outcomes and Measures. The primary efficacy endpoint was CRP PMR-AS< 10 (Min=0-Max 100 with higher values indicating greater activity, no MCID defined) combined with either prednisone ≤5 mg/day or a ≥10-mg prednisone decrease at week 24. There were 11 secondary outcomes assessed at week 24 included in this report, including disease activity and proportion of patients who were no longer taking prednisone.
Results: Of the 101 patients randomized (mean age, 67.2 years; 68 [67.3%] females), 100 (99%) received at least one infusion and 100% completed the trial. The primary endpoint was achieved in 67.3% and 31.4% of patients in the tocilizumab and placebo groups, respectively ( adjusted relative risk, 2.3; 95% confidence interval [95%CI]: 1.5 to 3.6; P< 0.001). Of 11 reported secondary endpoints at week 24, seven showed significant differences favoring tocilizumab including mean CRP PMR-AS score (7.5 [95% CI, 5.4 to 9.6] vs. 14.9 [95% CI, 11.4 to 18.4]; adjusted difference, 7.5 with 95%CI 3.8 to 11.2; P< 0.001) and proportion of prednisone-free patients (49.0% vs. 19.6%; adjusted relative risk, 2.5; 95%CI: 1.8 to 3.5; P< 0.001). The most frequent adverse events were infections, experienced by 23 (46.9%) patients in the tocilizumab group and 20 (39.2%) in the placebo group.
Conclusion: Among patients with active polymyalgia rheumatica despite prednisone therapy, tocilizumab, compared to a placebo, resulted in a significantly greater proportion of patients achieving a CPR PMR-AS< 10 with reduced prednisone requirements at week 24. However, additional trials are needed to confirm efficacy and to determine the balance of potential benefits and harms.


Disclosures: V. Devauchelle, Pfizer, Novartis, AbbVie/Abbott, Novartis, Bristol-Myers Squibb(BMS), Roche-Chugai, Galapados; G. CARVAJAL ALEGRIA, Roche-Chugai; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; C. Richez, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Galapados, GlaxoSmithKlein(GSK), Eli Lilly, Novartis, Pfizer; M. Truchetet, AbbVie/Abbott, Pfizer, Eli Lilly, Galapados; D. Wendling, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Pfizer, Roche, Amgen, Nordic Pharma, UCB, Novartis, Janssen, Eli Lilly, Sandoz, Galapados, Grunenthal; E. TOUSSIROT, None; a. Perdriger, None; j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; R. FELTEN, Novartis, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Nordic Pharma, Merck/MSD, MEDAC, Pfizer, Sanofi, UCB; B. Fautrel, Pfizer, Novartis, Roche, Sanofi-Aventis, SOBI, UCB; l. chiche, Novartis, Bristol-Myers Squibb(BMS); P. HILLIQUIN, None; C. Le Henaff, None; B. Dervieux, None; G. Direz, Novartis, Roche, Sanofi; I. Chary-Valckenaere, None; D. CORNEC, None; D. Guellec, None; T. MARHADOUR, None; E. Nowak, None; A. Saraux, None.

