Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1379: Association of HLA Alleles with Specific Auto-antibodies in an Ancestrally Diverse Population of Childhood Systemic Lupus Erythematosus (SLE)
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Linda Hiraki, MD, FRCPC, ScD
Clinician-Scientist
The Hospital for Sick Children
Toronto, ON, Canada
Abstract Poster Presenter(s)
Nicholas Gold1, Fangming Liao1, JingJing Cao1, Daniela Dominguez1, Andrea Knight2, Deborah Levy3 and Linda Hiraki4, 1The Hospital for Sick Children, Toronto, ON, Canada, 2The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 3Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 4The Hospital for Sick Children, Division of Rheumatology, Department of Paediatrics, University of Toronto, Genetics and Genome Biology, SickKids Research Institute, Toronto, ON, Canada
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease capable of affecting multiple organ systems. Genetic variants in the Human Leukocyte Antigen (HLA) are associated with autoimmune disease, as well as specific autoantibodies in SLE. Yet most studies to date have focused on adults of European ancestry with SLE. We aimed to test the association of HLA variants and specific autoantibody profiles in an ancestrally diverse cohort of children and adolescents with SLE.
Methods: Our study included patients from a pediatric Lupus clinic diagnosed, using criteria from the American College of Rheumatology (ACR) and/or the Systemic Lupus Collaborating Clinics (SLICC), and followed for SLE between 1985 and 2018. Participants were genotyped on the multi-ethnic MEGA or GSA array. HLA alleles were imputed with SNP2HLA on the Michigan imputation server multi-ethnic HLA reference panel. 42,924 variants were tested in the HLA region. We completed unsupervised clustering to group patients into two clusters based on the eight autoantibodies (anti-Cardiolipin, dsDNA, La, LAC, RF, RNP, Ro, Sm). Autoantibody status was classified as positive if the patient ever had a positive titer. Candidate analyses of 8 independent HLA variants previously shown to be associated with anti-Ro and anti-La were performed, as well as an HLA-wide association study for each of the autoantibodies and the clusters.
Results: Our study included n=450 patients with childhood SLE, 82.5% female (Table 1). Autoantibody positivity was largest for anti-dsDNA at 70% (Figure 1). For 3 of the 8 independent candidate HLA variant analyses, we demonstrated a significant association with anti-Ro (rs3130781 [OR: 1.7 (1.1-2.6); p=0.01]) and anti-La (HLA-DRB1*03:01[OR: 1.9 (1.1-3.4); p=0.02], rs2894252 [OR: 3.0 (1.4-6.6); p=0.02]) (Table 2). These variants associated stronger in Europeans compared to non-Europeans. HLA-wide analysis did not identify a significant allele for any of the individual specific autoantibodies, nor with cluster membership.
Conclusion: In a multi-ethnic population of children and adolescents with SLE, we replicated associations of HLA-alleles significantly associated with anti-Ro and anti-La antibodies. We also observed heterogeneity of the HLA and autoantibody associations by ancestry. HLA-wide analyses did not identify additional antibody specific HLA alleles. Additional studies in larger, multi-ancestral cohorts will be an important next step into validate these results, and identify ancestry specific HLA associations with autoantibodies in SLE.
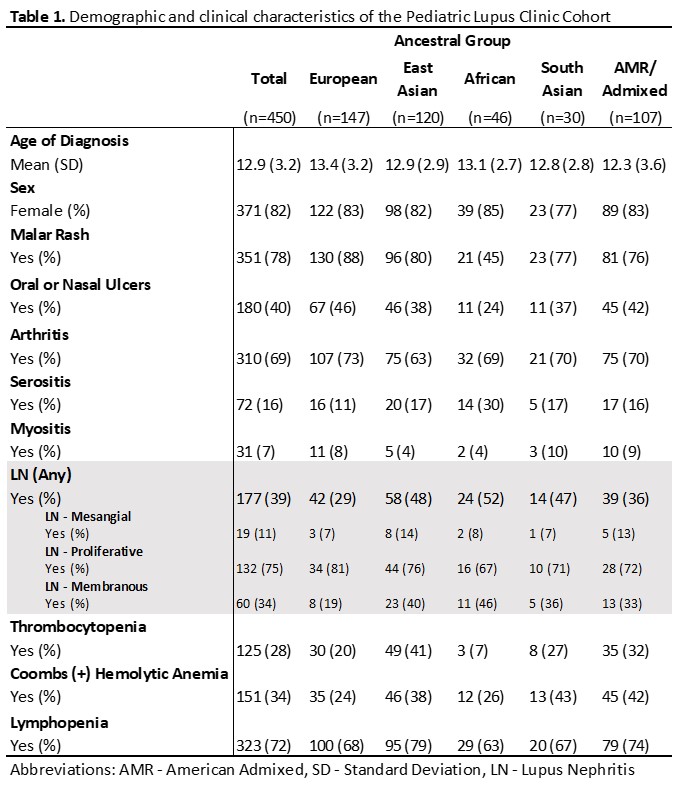 Table 1. Demographic and clinical characteristics of the Pediatric Lupus Clinic cohort
Table 1. Demographic and clinical characteristics of the Pediatric Lupus Clinic cohort
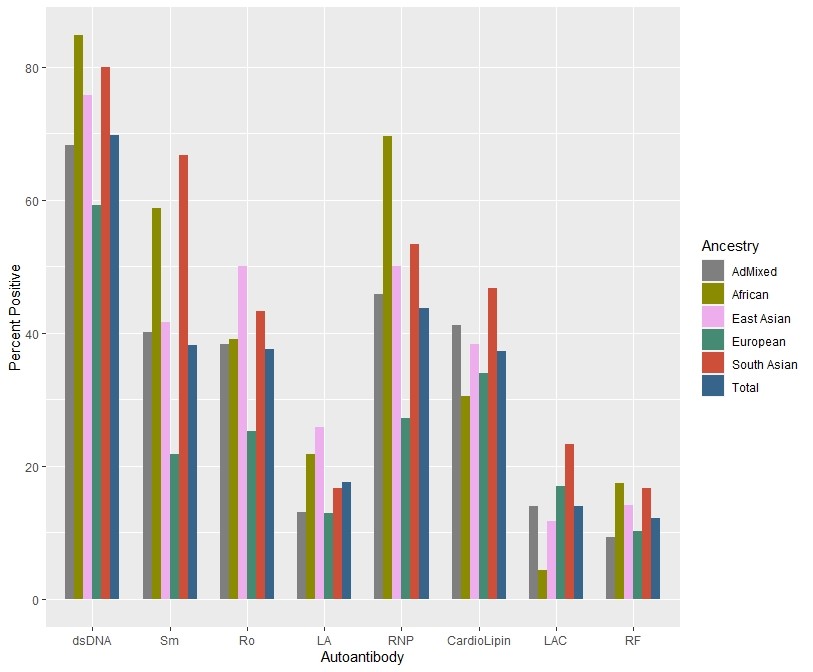 Figure 1. Autoantibody positivity of the pediatric Lupus Clinic cohort (n=450) stratified by genetically inferred ancestral group.
Figure 1. Autoantibody positivity of the pediatric Lupus Clinic cohort (n=450) stratified by genetically inferred ancestral group.
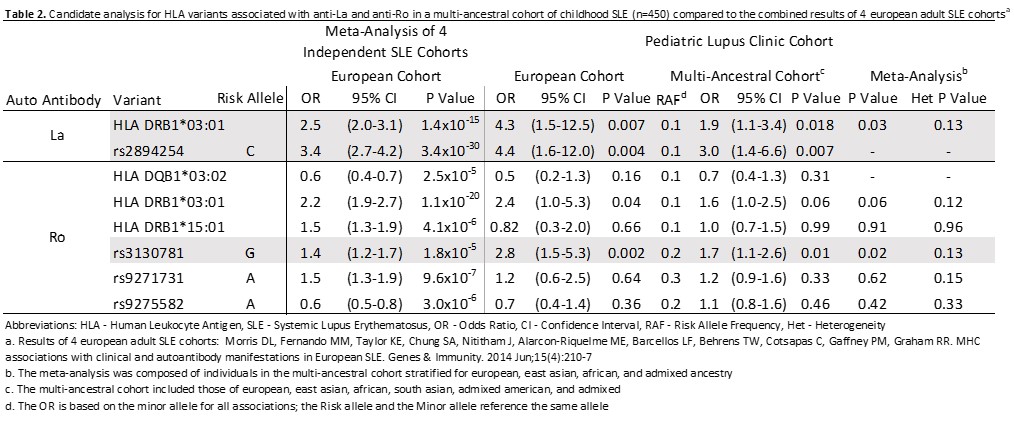 Table 2. Candidate analysis for HLA variants associated with anti-La and anti-Ro in a multi-ancestral cohort of childhood SLE (n=450) compared to the combined results of 4 European adult SLE cohorts
Table 2. Candidate analysis for HLA variants associated with anti-La and anti-Ro in a multi-ancestral cohort of childhood SLE (n=450) compared to the combined results of 4 European adult SLE cohorts
Disclosures: N. Gold, None; F. Liao, None; J. Cao, None; D. Dominguez, None; A. Knight, None; D. Levy, None; L. Hiraki, None.
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease capable of affecting multiple organ systems. Genetic variants in the Human Leukocyte Antigen (HLA) are associated with autoimmune disease, as well as specific autoantibodies in SLE. Yet most studies to date have focused on adults of European ancestry with SLE. We aimed to test the association of HLA variants and specific autoantibody profiles in an ancestrally diverse cohort of children and adolescents with SLE.
Methods: Our study included patients from a pediatric Lupus clinic diagnosed, using criteria from the American College of Rheumatology (ACR) and/or the Systemic Lupus Collaborating Clinics (SLICC), and followed for SLE between 1985 and 2018. Participants were genotyped on the multi-ethnic MEGA or GSA array. HLA alleles were imputed with SNP2HLA on the Michigan imputation server multi-ethnic HLA reference panel. 42,924 variants were tested in the HLA region. We completed unsupervised clustering to group patients into two clusters based on the eight autoantibodies (anti-Cardiolipin, dsDNA, La, LAC, RF, RNP, Ro, Sm). Autoantibody status was classified as positive if the patient ever had a positive titer. Candidate analyses of 8 independent HLA variants previously shown to be associated with anti-Ro and anti-La were performed, as well as an HLA-wide association study for each of the autoantibodies and the clusters.
Results: Our study included n=450 patients with childhood SLE, 82.5% female (Table 1). Autoantibody positivity was largest for anti-dsDNA at 70% (Figure 1). For 3 of the 8 independent candidate HLA variant analyses, we demonstrated a significant association with anti-Ro (rs3130781 [OR: 1.7 (1.1-2.6); p=0.01]) and anti-La (HLA-DRB1*03:01[OR: 1.9 (1.1-3.4); p=0.02], rs2894252 [OR: 3.0 (1.4-6.6); p=0.02]) (Table 2). These variants associated stronger in Europeans compared to non-Europeans. HLA-wide analysis did not identify a significant allele for any of the individual specific autoantibodies, nor with cluster membership.
Conclusion: In a multi-ethnic population of children and adolescents with SLE, we replicated associations of HLA-alleles significantly associated with anti-Ro and anti-La antibodies. We also observed heterogeneity of the HLA and autoantibody associations by ancestry. HLA-wide analyses did not identify additional antibody specific HLA alleles. Additional studies in larger, multi-ancestral cohorts will be an important next step into validate these results, and identify ancestry specific HLA associations with autoantibodies in SLE.
 Table 1. Demographic and clinical characteristics of the Pediatric Lupus Clinic cohort
Table 1. Demographic and clinical characteristics of the Pediatric Lupus Clinic cohort Figure 1. Autoantibody positivity of the pediatric Lupus Clinic cohort (n=450) stratified by genetically inferred ancestral group.
Figure 1. Autoantibody positivity of the pediatric Lupus Clinic cohort (n=450) stratified by genetically inferred ancestral group.  Table 2. Candidate analysis for HLA variants associated with anti-La and anti-Ro in a multi-ancestral cohort of childhood SLE (n=450) compared to the combined results of 4 European adult SLE cohorts
Table 2. Candidate analysis for HLA variants associated with anti-La and anti-Ro in a multi-ancestral cohort of childhood SLE (n=450) compared to the combined results of 4 European adult SLE cohortsDisclosures: N. Gold, None; F. Liao, None; J. Cao, None; D. Dominguez, None; A. Knight, None; D. Levy, None; L. Hiraki, None.

