Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1505: Blue-collar Workers with Psoriatic Arthritis: Disease Activity, Work Disability and Response to Biologics
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NC
Nina Colla
Department of Rheumatology, University Hospital Zurich, University of Zurich
Winterthur, Switzerland
Abstract Poster Presenter(s)
Nina Colla1, Julia-Tatjana Maul2, Burkhard Moeller3, Michael Nissen4, Nikhil Yawalkar5, Eleftherios Papagiannoulis6, Oliver Distler1, Adrian Ciurea7 and Raphael Micheroli8, 1Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 2Department of Dermatology and Venereology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 3Inselspital - University Hospital Bern, Bern, Switzerland, 4Hopitaux Universitaires de Genève, Geneva, Switzerland, 5Department of Dermatology, University Hospital Inselspital Bern, Bern, Switzerland, 6Statistics Group, SCQM Foundation, Zurich, Switzerland., Zürich, 7University Hospital Zurich, Zürich, Switzerland, 8University Hospital Zurich, Department of Rheumatology, Zürich, Switzerland
Background/Purpose: Biomechanical stress is a possible trigger of inflammation in psoriatic arthritis (PsA). The influence of physically demanding occupations on this potential association has not yet been investigated.
The aim of this study was to compare PsA patients who perform manual work (blue-collar (BCol) workers) with patients having more sedentary jobs (white-collar (WCol) workers) in terms of work disability, disease activity, and biologic response / retention rate.
Methods: PsA patients from the Swiss Clinical Quality Management cohort were included if they had available information on occupation and work disability, started a new biological treatment, and if baseline and follow-up (12 months ± 90 days) data on disease activity were available. Patients working in jobs associated with transportation, manufacturing or agriculture were classified as BCol, whereas patients working in less physically demanding jobs (office-worker, homemaker, teacher, etc.) were classified as WCol. Baseline characteristics were compared using Fisher's exact for categorical and Kruskal-Wilcoxon test for continuous variables.
For longitudinal analysis, patients with more than 4 weeks work disability were excluded. Generalized estimating equation (GEE) models were used to examine the proportion of patients who achieved DAS28-CRP remission at 1 year. The retention rate was assessed using Kaplan-Meier survival curves and multiple-adjusted Cox proportional hazard regression. The following potential confounders were included in the models: disease duration, baseline disease activity, sex, BMI, type and line of biologic treatment and csDMARD co-therapy.
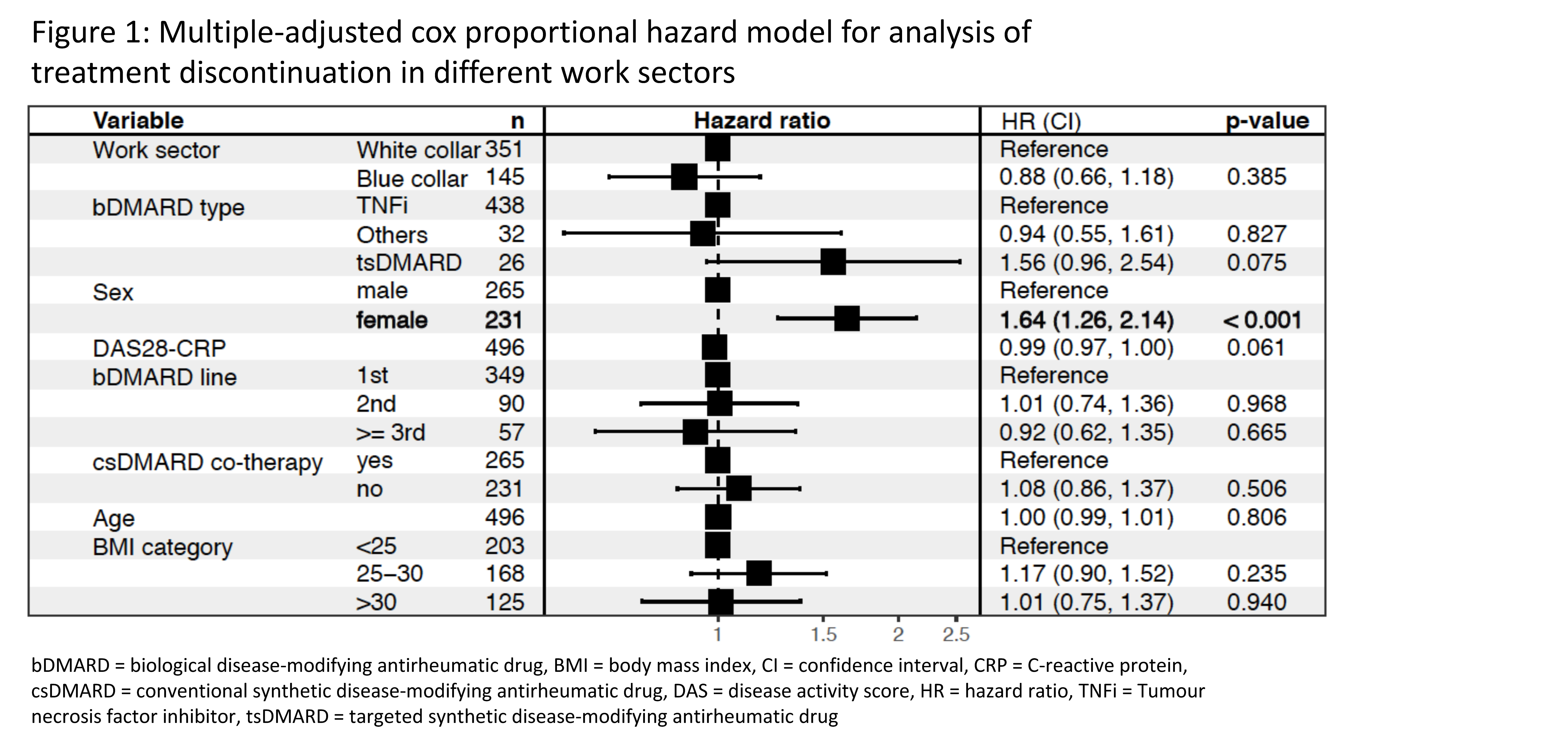
Results: 564 patients were included (29% BCol and 71% WCol worker). Baseline characteristics are shown in Table 1. At baseline, BCol workers were more likely to be male and had a higher rate of any work disability. Disease activity as well as patient reported outcomes were comparable. Occupation showed no effect on the proportion of patients achieving DAS28-CRP remission at one year in the GEE analysis (Table 2). Female sex and BMI > 30 kg/m2 were significantly associated with a lower remission rate in adjusted GEE analysis. Kaplan-Meier plots showed significantly longer biologic retention in BCol (mean retention duration 3.15 vs. 2.14 years in BCol and WCol, respectively). The adjusted retention analysis could not confirm this result, while the association remained for the female sex (Figure 1).
Conclusion: Compared with WCol workers, BCol workers with PsA had similar disease burden and response rates to biologics among those who continued to work. A very high percentage of BCol patients reported any work disability, which influences the true impact of work on PsA. However, work disability must be actively addressed in daily clinical practice, as employment is one of the most fundamental social determinants of health.
.jpg)
.jpg)
Disclosures: N. Colla, None; J. Maul, None; B. Moeller, None; M. Nissen, AbbVie/Abbott, Pfizer, Amgen, Novartis, Janssen; N. Yawalkar, None; E. Papagiannoulis, None; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Ciurea, AbbVie, Novartis, Merck/MSD; R. Micheroli, None.
Background/Purpose: Biomechanical stress is a possible trigger of inflammation in psoriatic arthritis (PsA). The influence of physically demanding occupations on this potential association has not yet been investigated.
The aim of this study was to compare PsA patients who perform manual work (blue-collar (BCol) workers) with patients having more sedentary jobs (white-collar (WCol) workers) in terms of work disability, disease activity, and biologic response / retention rate.
Methods: PsA patients from the Swiss Clinical Quality Management cohort were included if they had available information on occupation and work disability, started a new biological treatment, and if baseline and follow-up (12 months ± 90 days) data on disease activity were available. Patients working in jobs associated with transportation, manufacturing or agriculture were classified as BCol, whereas patients working in less physically demanding jobs (office-worker, homemaker, teacher, etc.) were classified as WCol. Baseline characteristics were compared using Fisher's exact for categorical and Kruskal-Wilcoxon test for continuous variables.
For longitudinal analysis, patients with more than 4 weeks work disability were excluded. Generalized estimating equation (GEE) models were used to examine the proportion of patients who achieved DAS28-CRP remission at 1 year. The retention rate was assessed using Kaplan-Meier survival curves and multiple-adjusted Cox proportional hazard regression. The following potential confounders were included in the models: disease duration, baseline disease activity, sex, BMI, type and line of biologic treatment and csDMARD co-therapy.
Results: 564 patients were included (29% BCol and 71% WCol worker). Baseline characteristics are shown in Table 1. At baseline, BCol workers were more likely to be male and had a higher rate of any work disability. Disease activity as well as patient reported outcomes were comparable. Occupation showed no effect on the proportion of patients achieving DAS28-CRP remission at one year in the GEE analysis (Table 2). Female sex and BMI > 30 kg/m2 were significantly associated with a lower remission rate in adjusted GEE analysis. Kaplan-Meier plots showed significantly longer biologic retention in BCol (mean retention duration 3.15 vs. 2.14 years in BCol and WCol, respectively). The adjusted retention analysis could not confirm this result, while the association remained for the female sex (Figure 1).
Conclusion: Compared with WCol workers, BCol workers with PsA had similar disease burden and response rates to biologics among those who continued to work. A very high percentage of BCol patients reported any work disability, which influences the true impact of work on PsA. However, work disability must be actively addressed in daily clinical practice, as employment is one of the most fundamental social determinants of health.
.jpg)
.jpg)

Disclosures: N. Colla, None; J. Maul, None; B. Moeller, None; M. Nissen, AbbVie/Abbott, Pfizer, Amgen, Novartis, Janssen; N. Yawalkar, None; E. Papagiannoulis, None; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Ciurea, AbbVie, Novartis, Merck/MSD; R. Micheroli, None.

