Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1525: Breakthrough Infections in COVID-19 Vaccinated Patients with Systemic Sclerosis: A Survival Analysis from a Multicenter International Patient-Reported Survey
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SA
Sakir Ahmed, DM
Kalinga Institute of Medical Sciences
Bhubaneswar, Odisha, India
Abstract Poster Presenter(s)
Sakir Ahmed1, Naveen R2, John Pauling3, Darpan Thakare2, Chris Wincup4, Nicoletta Del Papa5, Gianluca Sambataro6, Fabiola Atzeni7, SIMONE PARISI8, Marcello Govoni9, Elena Bartoloni Bocci10, Gian Domenico Sebastiani11, Enrico Fusaro12, Marco Sebastiani13, Luca Quartuccio14, Franco Franceschini15, Pier Paolo Sainaghi16, Giovanni Orsolini17, Rossella De Angelis18, Maria Giovanna Danielli19, Vincenzo Venerito20, Parikshit Sen21, Minchul Kim22, Abraham Edgar Gracia-Ramos23, Akira Yoshida24, James B. Lilleker25, Vishwesh Agarwal26, Sinan Kardes27, Jessica Day28, Mrudula Joshi29, Marcin Milchert30, Tamer A Gheita31, Babur Salim32, Ioannis Parodis33, Albert Selva O’Callaghan34, Elena Nikiphorou35, Tulika Chatterjee22, Ai Lyn Tan36, Arvind Nune37, Lorenzo Cavagna38, Samuel Shinjo39, Nelly Ziade40, Johannes Knitza41, Hector Chinoy42, Oliver Distler43, Masataka Kuwana44, Rohit Aggarwal45, Latika Gupta46, Vikas Agarwal2 and Ashima Makol47, 1Kalinga Institute of Medical Sciences, Bhubaneswar, India, 2Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 3North Bristol NHS Trust, Bristol, United Kingdom, 4University College London, London, United Kingdom, 5Unità operativa complessa (UOC) Day Hospital Reumatologia via Gaetano Pini 9, Centro Specialistico Ortopedico Traumatologico, Gaetano Pini-CTO, Milano, Italy, 6Medico Immunologia e reumatologia presso, Artoreuma S.R.L., Cors S, Mascalucia, 7Rheumatology Unit, University of Messina, Messina, Italy, 8Italian Society for Rheumatology, Turin, Italy, 9S. Anna Hospital and University of Ferrara, Ferrara, Italy, 10Rheumatology Unit. Department of Medicine, Perugia, Perugia, Italy, 11U.O.C. Reumatologia, Ospedale San Camillo-Forlanini,, Roma, 12Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Turin, Italy, 13Azienda Policlinico di Modena, Modena, Italy, 14Clinic of Rheumatology, Department of Medicine (DAME), ASUFC, University of Udine, Udine, Italy, 15Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Brescia, Italy, 16Interdisciplinary Research Center of Autoimmune Diseases, Novara, Italy, 17Department of Medicine, Rheumatology Unit, University of Verona, Verona, Verona, 18Rheumatology Unit, Department of Clinical and Molecular Sciences, Polytechnic University of Marche, Ancona, Italy, 19Dipartimento di Scienze Cliniche e Molecolari, Università Politecnica delle, Marche, Italy, 20Department of Emergency and Organ Transplantations-Rheumatology Unit, University of Bari "Aldo Moro", Bari, Italy, 21Maulana Azad Medical College, New Delhi, India, 22University of Illinois College of Medicine Peoria, Peoria, IL, 23Instituto Mexicano del Seguro Social, Ciudad de México, Mexico, 24Nippon Medical School Graduate School of Medicine, Bunkyo-ku, Tokyo, Japan, 25The University of Manchester, Manchester, United Kingdom, 26Mahatma Gandhi Missions Medical College, Lucknow, India, 27Istanbul University, Istanbul, Turkey, 28Walter and Eliza Hall Institute, Melbourne, Australia, 29Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India, 30Pomeranian Medical University in Szczecin, Szczecin, Poland, 31Rheumatology Department, Faculty of Medicine, Cairo University, Cairo, Egypt, 32Fauji foundation hospital Rawalpindi, Rawalpindi, Pakistan, 33Karolinska Institutet, Stockholm, Sweden, 34Hospital Universitari Vall d'Hebron, Barcelona, Spain, 35Leiden University Medical Center & King's College London, London, United Kingdom, 36University of Leeds, Leeds, United Kingdom, 37Southport and Ormskirk Hospital NHS Trust, Southport, United Kingdom, 38Università di Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, Pavia, Italy, 39Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil, 40Saint-Joseph University, Beirut, Lebanon, 41Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-UniversityErlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany, 42The University of Manchester, Sale, United Kingdom, 43Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 44Nippon Medical School Graduate School of Medicine, Tokyo, Japan, 45Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, 46Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom, 47Mayo Clinic, Rochester, MN, Rochester, MN
Background/Purpose: Patients with systemic autoimmune rheumatic diseases (AIRDs) are considered more susceptible to break through infection (BI) following vaccination due to their immunosuppressed status and associated co-morbid conditions. Data on BI in patients with systemic sclerosis (SSc) is scarce. These patients are at higher risks of adverse outcomes from COVID-19 due to high prevalence of interstitial lung disease and cardiovascular co-morbidities.
Methods: Data on respondents with SSc, non-SSc AIRDs and healthy controls (HCs) was extracted from the COVAD database, an international self-reported SurveyMonkey platform based online survey that captured respondent demographics, comorbidities, AIRD characteristics, COVID-19 infection history, and COVID-19 Vaccination details. Fully vaccinated patients (completed 2 doses of vaccine) who did not report COVID-19 prior to vaccination were included. BI was defined as per the CDC definition. Frequency of BI, symptoms, duration of illness, and severity (hospitalization or supplementary oxygen) were compared between the groups.
The survival analysis included Kaplan Meier curves with log-rank tests. Cox proportionate regression was used to assess the association of age, gender, ethnicity and immunosuppressive drugs at the time of vaccination on BI.
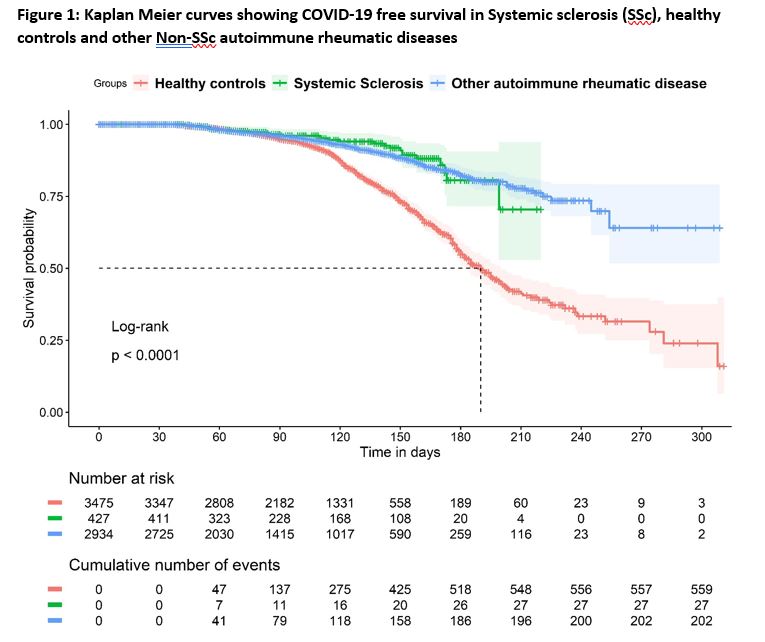
Results: Of 10900 respondents in the COVAD database, 6836 fulfilling inclusion criteria [SSc (n=427), other AIRDs (n=2934) and HCs (n=3475)] were analysed. BI were reported in 27 (6.3%) of SSc, 202 (6.9%) non-SSc AIRD and 560 (16.1%) of HCs during a median follow up of 100 days (IQR: 60-137) since the first dose of vaccination.
The duration and symptomatology of BI is compared between the three groups in Table-1. Hospitalization [SSc:4 (14.8%); HC:37 (6.61%); non SSc AIRD: 32(15.8%)] and the need for oxygenation [SSc:1 (25%); HC:17 (45.9%); non SSc AIRD: 13(40.6%)] were statistically same between the groups.
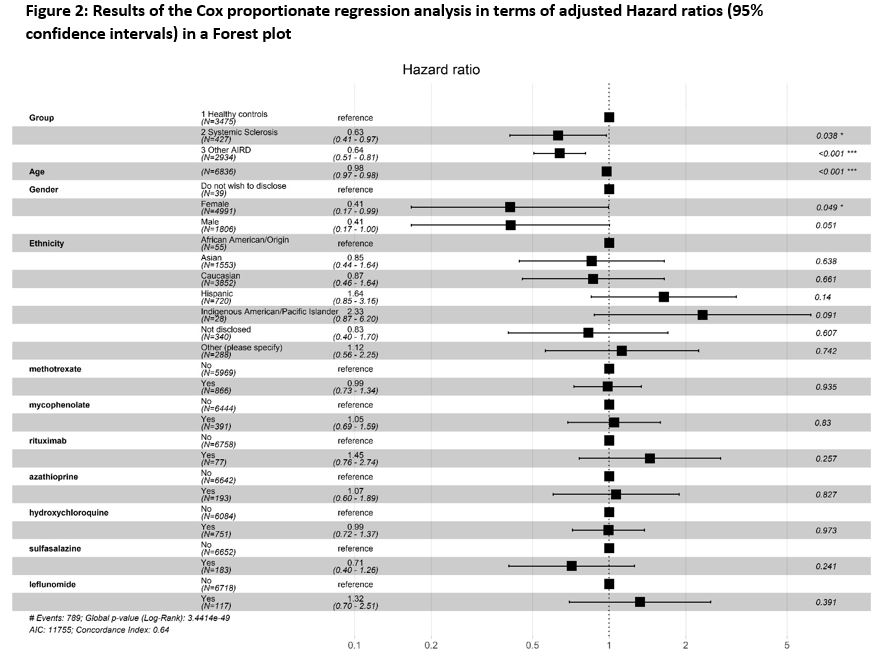
The incidence of BI in SSc was lower than that of HC, but comparable to non-SSc AIRD [Figure-1]. SSc had lower risk for BI [Hazard ratio (HR): 0.56 (95%CI: 0.46-0.74)]. BIs were associated with age [HR: 0.98 (0.97-0.98)] but not ethnicities or immunosuppressive drugs at the time of vaccination [Figure 2].
Conclusion: Patients with SSc had lower risk for BI as compared to HC, but similar to that in non-SSc AIRD. This may indicate that people with SSc and other AIRDs have continued to take effective precautions against contracting COVID-19. Severity of BI was not different between groups. Advancing age, but not ethnicity or immunosuppressive medication were associated with BIs.
.jpg)
Disclosures: S. Ahmed, DrReddy, Novartis, Pfizer, Janssen, Cipla; N. R, None; J. Pauling, None; D. Thakare, None; C. Wincup, None; N. Del Papa, None; G. Sambataro, None; F. Atzeni, None; S. PARISI, None; M. Govoni, None; E. Bartoloni Bocci, None; G. Sebastiani, None; E. Fusaro, None; M. Sebastiani, None; L. Quartuccio, None; F. Franceschini, None; P. Sainaghi, None; G. Orsolini, None; R. De Angelis, None; M. Giovanna Danielli, None; V. Venerito, None; P. Sen, None; M. Kim, None; A. Gracia-Ramos, None; A. Yoshida, None; J. Lilleker, None; V. Agarwal, None; S. Kardes, None; J. Day, CSL; M. Joshi, None; M. Milchert, None; T. Gheita, None; B. Salim, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche; A. O’Callaghan, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; T. Chatterjee, None; A. Tan, None; A. Nune, None; L. Cavagna, None; S. Shinjo, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; H. Chinoy, Eli Lilly, UCB; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Gupta, None; V. Agarwal, None; A. Makol, Boehringer-Ingelheim.
Background/Purpose: Patients with systemic autoimmune rheumatic diseases (AIRDs) are considered more susceptible to break through infection (BI) following vaccination due to their immunosuppressed status and associated co-morbid conditions. Data on BI in patients with systemic sclerosis (SSc) is scarce. These patients are at higher risks of adverse outcomes from COVID-19 due to high prevalence of interstitial lung disease and cardiovascular co-morbidities.
Methods: Data on respondents with SSc, non-SSc AIRDs and healthy controls (HCs) was extracted from the COVAD database, an international self-reported SurveyMonkey platform based online survey that captured respondent demographics, comorbidities, AIRD characteristics, COVID-19 infection history, and COVID-19 Vaccination details. Fully vaccinated patients (completed 2 doses of vaccine) who did not report COVID-19 prior to vaccination were included. BI was defined as per the CDC definition. Frequency of BI, symptoms, duration of illness, and severity (hospitalization or supplementary oxygen) were compared between the groups.
The survival analysis included Kaplan Meier curves with log-rank tests. Cox proportionate regression was used to assess the association of age, gender, ethnicity and immunosuppressive drugs at the time of vaccination on BI.
Results: Of 10900 respondents in the COVAD database, 6836 fulfilling inclusion criteria [SSc (n=427), other AIRDs (n=2934) and HCs (n=3475)] were analysed. BI were reported in 27 (6.3%) of SSc, 202 (6.9%) non-SSc AIRD and 560 (16.1%) of HCs during a median follow up of 100 days (IQR: 60-137) since the first dose of vaccination.
The duration and symptomatology of BI is compared between the three groups in Table-1. Hospitalization [SSc:4 (14.8%); HC:37 (6.61%); non SSc AIRD: 32(15.8%)] and the need for oxygenation [SSc:1 (25%); HC:17 (45.9%); non SSc AIRD: 13(40.6%)] were statistically same between the groups.
The incidence of BI in SSc was lower than that of HC, but comparable to non-SSc AIRD [Figure-1]. SSc had lower risk for BI [Hazard ratio (HR): 0.56 (95%CI: 0.46-0.74)]. BIs were associated with age [HR: 0.98 (0.97-0.98)] but not ethnicities or immunosuppressive drugs at the time of vaccination [Figure 2].
Conclusion: Patients with SSc had lower risk for BI as compared to HC, but similar to that in non-SSc AIRD. This may indicate that people with SSc and other AIRDs have continued to take effective precautions against contracting COVID-19. Severity of BI was not different between groups. Advancing age, but not ethnicity or immunosuppressive medication were associated with BIs.
.jpg)


Disclosures: S. Ahmed, DrReddy, Novartis, Pfizer, Janssen, Cipla; N. R, None; J. Pauling, None; D. Thakare, None; C. Wincup, None; N. Del Papa, None; G. Sambataro, None; F. Atzeni, None; S. PARISI, None; M. Govoni, None; E. Bartoloni Bocci, None; G. Sebastiani, None; E. Fusaro, None; M. Sebastiani, None; L. Quartuccio, None; F. Franceschini, None; P. Sainaghi, None; G. Orsolini, None; R. De Angelis, None; M. Giovanna Danielli, None; V. Venerito, None; P. Sen, None; M. Kim, None; A. Gracia-Ramos, None; A. Yoshida, None; J. Lilleker, None; V. Agarwal, None; S. Kardes, None; J. Day, CSL; M. Joshi, None; M. Milchert, None; T. Gheita, None; B. Salim, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche; A. O’Callaghan, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; T. Chatterjee, None; A. Tan, None; A. Nune, None; L. Cavagna, None; S. Shinjo, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; H. Chinoy, Eli Lilly, UCB; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Gupta, None; V. Agarwal, None; A. Makol, Boehringer-Ingelheim.

