Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1417: Clinical and Demographic Characteristics of Patients with RF+/ACPA+ RA and First-Line TNF Inhibitor versus Abatacept Treatment Choice in Real-World Clinical Practice
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LN
Laetitia N'Dri, PharmD
BMS
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Gordon Lam1, Hanke Zheng2, Emily Bland3, Vardhaman Patel4, Laetitia N’Dri5, Parisa Asgarisabet3, Keith Wittstock4, Cherrishe Brown-Bickerstaff3, Mark Chaballa2, Bruce Feinberg3, Vadim Khaychuk6 and Andrew J Klink3, 1Arthritis & Osteoporosis Consultants of the Carolinas, Charlotte, NC, 2Bristol Myers Squibb, Princeton, NJ, 3Cardinal Health, Dublin, OH, 4Bristol Myers Squibb, Lawrence Township, NJ, 5Bristol Myers Squibb, Princeton, 6Bristol Myers Squibb, Pennington, MA
Background/Purpose: Early intensive treatment (tx) is an accepted paradigm in the tx of patients (pts) with RA with poor prognostic factors (eg, RF/ACPA seropositivity); however, guidelines for optimal biologic DMARD (bDMARD) selection and sequencing following inadequate response to conventional DMARDs (cDMARDs) are not established. Understanding differences in real-world (RW) pts with RA and factors associated with first-line (1L) bDMARD selection will provide context for interpreting data on relative effectiveness. This study compares pts with dual-seropositive (RF+/ACPA+) RA at 1L TNF inhibitor (TNFi) or abatacept (ABA) initiation and identifies factors associated with 1L bDMARD selection.
Methods: In this retrospective cohort study, chart data were abstracted from pts with RF+/ACPA+ RA who were biologic- and Janus kinase inhibitor-tx naive and who initiated 1L ABA or TNFi between 1/2015 and 11/2020 after inadequate response to MTX. Pt characteristics were compared across cohorts using chi-square/Fisher exact tests for categorical variables and t-/Wilcoxon tests for continuous variables. Adjusted odds of receiving TNFi vs ABA were estimated using 2-level logistic regression.
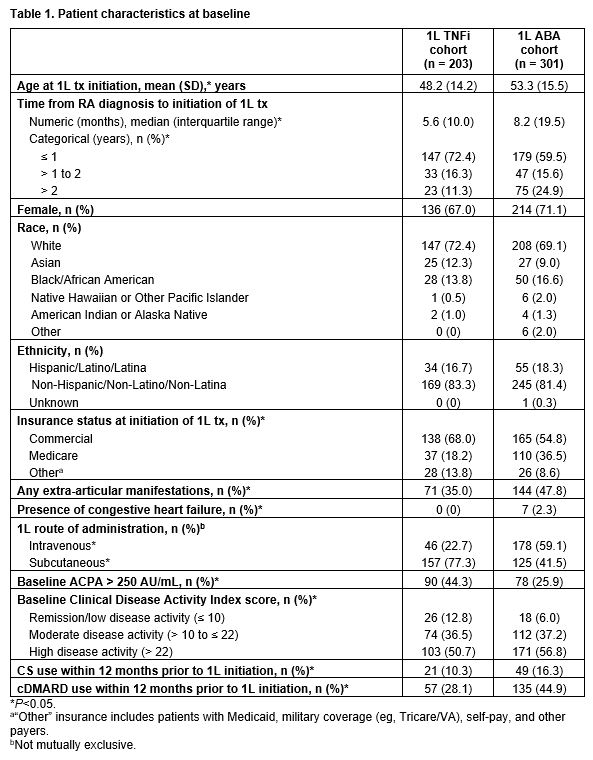
Results: Race, ethnicity, and sex were similar across cohorts (Table 1). Compared with TNFi pts (n = 203), ABA pts (n = 301) were older at 1L initiation (mean age [standard deviation], 53.3 [15.5] vs 48.2 [14.2] years), had longer time from diagnosis to 1L initiation (median time [interquartile range], 8.2 [19.5] vs 5.6 [10.0] months), and were more likely to have Medicare insurance (36.5% vs 18.2%), extra-articular manifestations (47.8% vs 35.0%), heart failure (2.3% vs. 0%), and to have received 1L tx intravenously (59.1% vs 22.7%; all P < 0.01; Table 1). ABA pts were more likely to have lower (≤ 250 AU/mL) ACPA levels (74.1% vs 55.7%; P < 0.01), high disease activity (Clinical Disease Activity Index > 22: 56.8% vs 50.7%; P = 0.03), and to have received CS and cDMARDs within 12 months prior to 1L initiation (16.3% vs 10.3%; P = 0.01 and 44.9% vs 28.1%; P < 0.01, respectively; Table 1).
Significantly higher odds (odds ratio [95% confidence interval]) of receiving ABA vs TNFi were associated with > 2 years from diagnosis to 1L initiation (2.9 [1.4–6.1]), moderate and high disease activity (4.2 [1.5–11.6] and 5.0 [1.8–13.9], respectively), and cDMARD use within 12 months prior to initiation (2.0 [1.1–3.6]) (all P < 0.05). Compared with those with commercial coverage, pts with Medicare/other insurance had increased odds of receiving ABA as age increased. ACPA > 250 AU/mL was associated with lower odds of receiving ABA (0.4 [0.2–0.8]; P < 0.01).
Conclusion: The results indicate that physicians prefer to prescribe abatacept over TNFi for RF/ACPA+ pts with higher disease severity, older age, the presence of extra-articular manifestations, and comorbidities such as heart failure. Our study highlights the need to adjust for baseline characteristics, such as articular and extra articular manifestations, between treatment groups in future comparative analysis.
Editorial support: Danielle Johnson (Caudex), funded by Bristol Myers Squibb.
Disclosures: G. Lam, AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, UCB, AstraZeneca, Sanofi, Aurinia, Horizon; H. Zheng, Bristol Myers Squibb; E. Bland, Cardinal Health; V. Patel, Bristol Myers Squibb; L. N’Dri, Bristol Myers Squibb; P. Asgarisabet, Cardinal Health; K. Wittstock, Bristol Myers Squibb; C. Brown-Bickerstaff, Cardinal Health; M. Chaballa, Bristol Myers Squibb; B. Feinberg, Cardinal Health; V. Khaychuk, Bristol Myers Squibb; A. Klink, Cardinal Health.
Background/Purpose: Early intensive treatment (tx) is an accepted paradigm in the tx of patients (pts) with RA with poor prognostic factors (eg, RF/ACPA seropositivity); however, guidelines for optimal biologic DMARD (bDMARD) selection and sequencing following inadequate response to conventional DMARDs (cDMARDs) are not established. Understanding differences in real-world (RW) pts with RA and factors associated with first-line (1L) bDMARD selection will provide context for interpreting data on relative effectiveness. This study compares pts with dual-seropositive (RF+/ACPA+) RA at 1L TNF inhibitor (TNFi) or abatacept (ABA) initiation and identifies factors associated with 1L bDMARD selection.
Methods: In this retrospective cohort study, chart data were abstracted from pts with RF+/ACPA+ RA who were biologic- and Janus kinase inhibitor-tx naive and who initiated 1L ABA or TNFi between 1/2015 and 11/2020 after inadequate response to MTX. Pt characteristics were compared across cohorts using chi-square/Fisher exact tests for categorical variables and t-/Wilcoxon tests for continuous variables. Adjusted odds of receiving TNFi vs ABA were estimated using 2-level logistic regression.
Results: Race, ethnicity, and sex were similar across cohorts (Table 1). Compared with TNFi pts (n = 203), ABA pts (n = 301) were older at 1L initiation (mean age [standard deviation], 53.3 [15.5] vs 48.2 [14.2] years), had longer time from diagnosis to 1L initiation (median time [interquartile range], 8.2 [19.5] vs 5.6 [10.0] months), and were more likely to have Medicare insurance (36.5% vs 18.2%), extra-articular manifestations (47.8% vs 35.0%), heart failure (2.3% vs. 0%), and to have received 1L tx intravenously (59.1% vs 22.7%; all P < 0.01; Table 1). ABA pts were more likely to have lower (≤ 250 AU/mL) ACPA levels (74.1% vs 55.7%; P < 0.01), high disease activity (Clinical Disease Activity Index > 22: 56.8% vs 50.7%; P = 0.03), and to have received CS and cDMARDs within 12 months prior to 1L initiation (16.3% vs 10.3%; P = 0.01 and 44.9% vs 28.1%; P < 0.01, respectively; Table 1).
Significantly higher odds (odds ratio [95% confidence interval]) of receiving ABA vs TNFi were associated with > 2 years from diagnosis to 1L initiation (2.9 [1.4–6.1]), moderate and high disease activity (4.2 [1.5–11.6] and 5.0 [1.8–13.9], respectively), and cDMARD use within 12 months prior to initiation (2.0 [1.1–3.6]) (all P < 0.05). Compared with those with commercial coverage, pts with Medicare/other insurance had increased odds of receiving ABA as age increased. ACPA > 250 AU/mL was associated with lower odds of receiving ABA (0.4 [0.2–0.8]; P < 0.01).
Conclusion: The results indicate that physicians prefer to prescribe abatacept over TNFi for RF/ACPA+ pts with higher disease severity, older age, the presence of extra-articular manifestations, and comorbidities such as heart failure. Our study highlights the need to adjust for baseline characteristics, such as articular and extra articular manifestations, between treatment groups in future comparative analysis.
Editorial support: Danielle Johnson (Caudex), funded by Bristol Myers Squibb.

Disclosures: G. Lam, AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, UCB, AstraZeneca, Sanofi, Aurinia, Horizon; H. Zheng, Bristol Myers Squibb; E. Bland, Cardinal Health; V. Patel, Bristol Myers Squibb; L. N’Dri, Bristol Myers Squibb; P. Asgarisabet, Cardinal Health; K. Wittstock, Bristol Myers Squibb; C. Brown-Bickerstaff, Cardinal Health; M. Chaballa, Bristol Myers Squibb; B. Feinberg, Cardinal Health; V. Khaychuk, Bristol Myers Squibb; A. Klink, Cardinal Health.

