Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1267–1303) Measures and Measurement of Healthcare Quality Poster
1284: Comparative Effectiveness Randomized Controlled Trials in Rheumatology Guidelines
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MP
Mike Putman, MD, MSc
The Medical College of Wisconsin
Milwaukee, WI, United States
Abstract Poster Presenter(s)
Kathryn Henry1, Desh Nepal2, Erin Valley2, Connor Pedersen2, Ali Duarte-Garcia3 and Mike Putman4, 1Medical College of Wisconsin, Manitowoc, WI, 2Medical College of Wisconsin, Milwaukee, WI, 3Mayo Clinic, Rochester, MN, 4The Medical College of Wisconsin, Milwaukee, WI
Background/Purpose: Comparative effectiveness randomized controlled trials (RCTs) compare two active interventions in a head-to-head design. They are useful for informing clinical practice guidelines, but the degree to which such trials inform clinical practice guidelines in rheumatology is unknown.
Methods: The American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) websites were searched from 1/1/2017-6/12/2021 for current clinical practice guidelines. RCTs referenced by each guideline were identified, and information regarding design and outcomes were extracted. Clinical practice recommendations from each guideline were also analyzed.
Results: Fifteen ACR and 9 EULAR endorsed guidelines were included, which cited 609 RCTs and provided 481 recommendations. Referenced RCTs enrolled an average of 418 patients (SD 985), most frequently evaluated biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) (70.1%), and infrequently utilized a head-to-head design (28%). A minority of recommendations received a high level of evidence (LOE) by the GRADE methodology (2.9%) or an "A" grade by the OCEBM methodology (28.9%). LOE was higher for recommendations informed by RCTs (p < 0.001) or head-to-head RCTs (p = 0.008). Many recommendations received a strong recommendation despite low (8, 2.6%) or very low (25, 8.3%) LOE.
Conclusion: Less than 1 in 6 rheumatology guideline recommendations are informed by head-to-head RCTs. Recommendations that were informed by head-to-head RCTs were more likely to have a high LOE by both GRADE and OCEBM. Efforts to introduce more comparative effectiveness RCTs should be undertaken.
.jpeg) Figure 1 (a): Percent of recommendations that are informed by H2H RCT.
Figure 1 (a): Percent of recommendations that are informed by H2H RCT.
Figure 1 (b) Level of evidence and strength of recommendations.
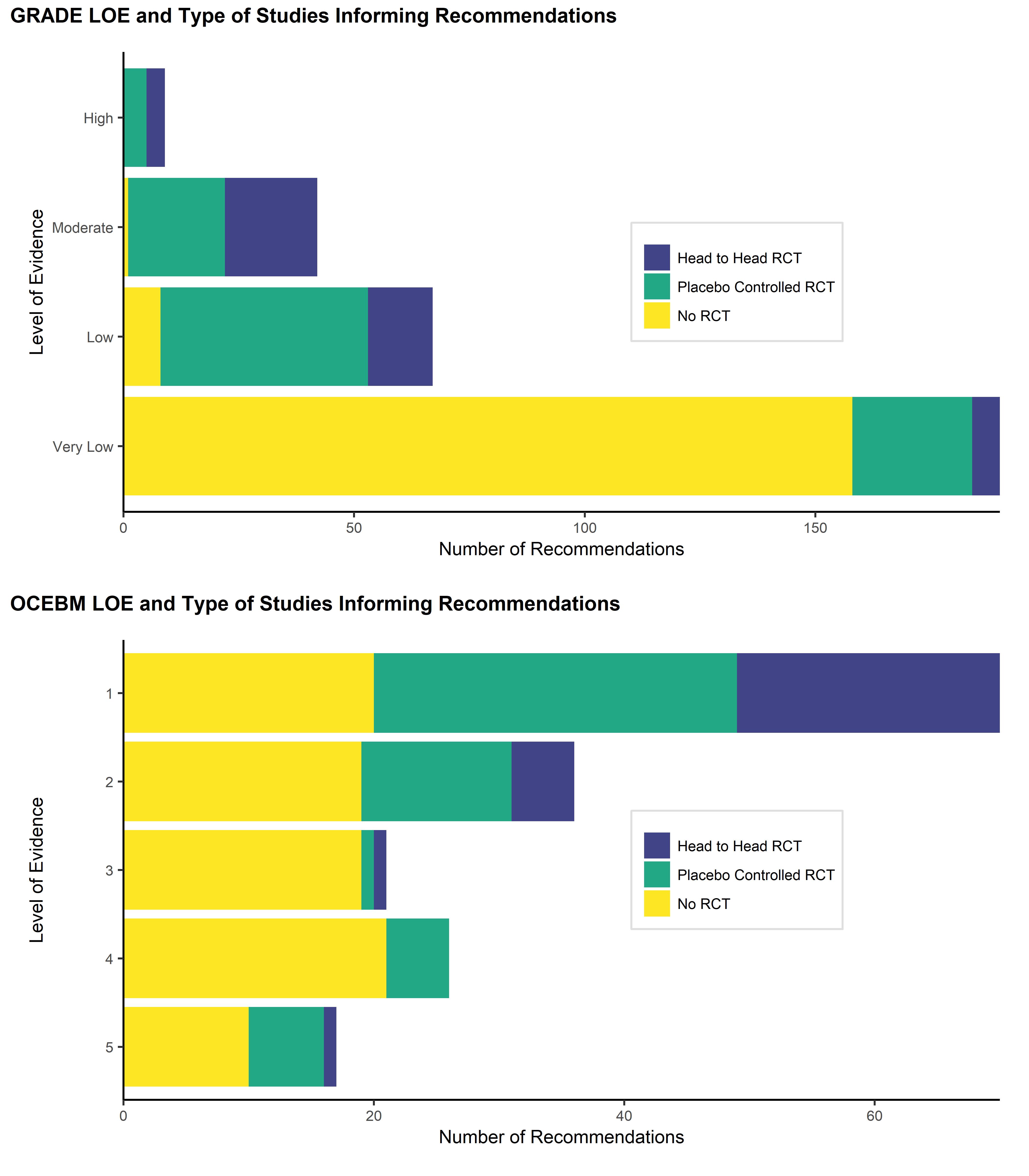 Figure 2 (a): GRADE LOE and type of studies informing recommendations
Figure 2 (a): GRADE LOE and type of studies informing recommendations
Figure 2(b): OCEBM LOE and type studies informing recommendations
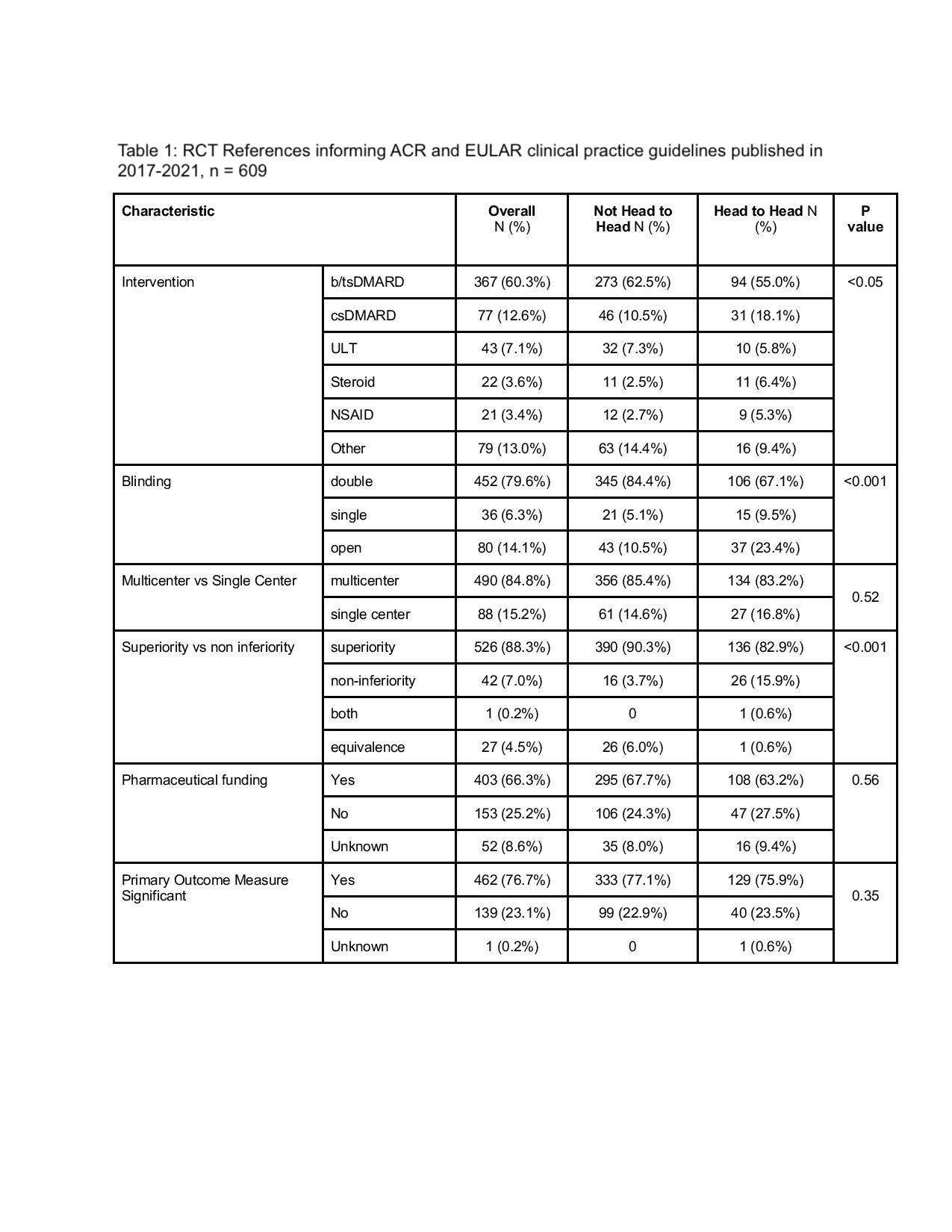 Table 1: RCT References informing ACR and EULAR clinical practice guidelines published in 2017-2021, n = 609
Table 1: RCT References informing ACR and EULAR clinical practice guidelines published in 2017-2021, n = 609
Disclosures: K. Henry, None; D. Nepal, None; E. Valley, None; C. Pedersen, None; A. Duarte-Garcia, None; M. Putman, AstraZeneca, AbbVie/Abbott, Novartis.
Background/Purpose: Comparative effectiveness randomized controlled trials (RCTs) compare two active interventions in a head-to-head design. They are useful for informing clinical practice guidelines, but the degree to which such trials inform clinical practice guidelines in rheumatology is unknown.
Methods: The American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) websites were searched from 1/1/2017-6/12/2021 for current clinical practice guidelines. RCTs referenced by each guideline were identified, and information regarding design and outcomes were extracted. Clinical practice recommendations from each guideline were also analyzed.
Results: Fifteen ACR and 9 EULAR endorsed guidelines were included, which cited 609 RCTs and provided 481 recommendations. Referenced RCTs enrolled an average of 418 patients (SD 985), most frequently evaluated biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) (70.1%), and infrequently utilized a head-to-head design (28%). A minority of recommendations received a high level of evidence (LOE) by the GRADE methodology (2.9%) or an "A" grade by the OCEBM methodology (28.9%). LOE was higher for recommendations informed by RCTs (p < 0.001) or head-to-head RCTs (p = 0.008). Many recommendations received a strong recommendation despite low (8, 2.6%) or very low (25, 8.3%) LOE.
Conclusion: Less than 1 in 6 rheumatology guideline recommendations are informed by head-to-head RCTs. Recommendations that were informed by head-to-head RCTs were more likely to have a high LOE by both GRADE and OCEBM. Efforts to introduce more comparative effectiveness RCTs should be undertaken.
.jpeg) Figure 1 (a): Percent of recommendations that are informed by H2H RCT.
Figure 1 (a): Percent of recommendations that are informed by H2H RCT.Figure 1 (b) Level of evidence and strength of recommendations.
 Figure 2 (a): GRADE LOE and type of studies informing recommendations
Figure 2 (a): GRADE LOE and type of studies informing recommendations Figure 2(b): OCEBM LOE and type studies informing recommendations
 Table 1: RCT References informing ACR and EULAR clinical practice guidelines published in 2017-2021, n = 609
Table 1: RCT References informing ACR and EULAR clinical practice guidelines published in 2017-2021, n = 609Disclosures: K. Henry, None; D. Nepal, None; E. Valley, None; C. Pedersen, None; A. Duarte-Garcia, None; M. Putman, AstraZeneca, AbbVie/Abbott, Novartis.

