Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1531: Continued Treatment with Nintedanib in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): Three-Year Data from SENSCIS-ON
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YA
Yannick Allanore, MD
Université Paris Cité
Paris, France
Abstract Poster Presenter(s)
Yannick Allanore1, Madelon Vonk2, Oliver Distler3, Arata Azuma4, Maureen Mayes5, Alexandra James6, Veronika Kohlbrenner7, Margarida Alves8, Dinesh Khanna9 and Kristin Highland10, 1Department of Rheumatology A, Descartes University, APHP, Cochin Hospital, Paris, France, Paris, France, 2Department of Rheumatology, Radboud University Medical Center, Nijmegen, Netherlands, 3Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 4Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan, 5Division of Rheumatology and Clinical Immunogenetics, University of Texas McGovern Medical School, Houston, TX, 6Elderbrook solutions GmbH, Bietigheim-Bissingen, Germany, Bietigheim-Bissingen, Germany, 7Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, Connecticut, USA, Ridgefield, CT, 8Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany, 9Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI, 10Cleveland Clinic, Cleveland, Ohio, USA, Cleveland, OH
Background/Purpose: In the randomized placebo-controlled SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) (mL/year) over 52 weeks by 44%, with adverse events that were manageable for most patients. SENSCIS-ON is an open-label extension study investigating adverse events and decline in FVC in patients with SSc-ILD treated with nintedanib over the longer term.
Methods: In the SENSCIS trial, patients received nintedanib or placebo until the last patient reached week 52 but for ≤100 weeks. Patients with SSc-ILD who completed the SENSCIS trial or a drug–drug interaction (DDI) study of nintedanib and oral contraceptive in which female patients with SSc-ILD received nintedanib for ≥14 days to approximately 28 days were eligible to enter SENSCIS-ON. We analyzed adverse events and changes from baseline in FVC (mL) over 148 weeks in patients who received nintedanib in SENSCIS and continued nintedanib in SENSCIS-ON ("continued nintedanib" group) and in patients who received placebo in SENSCIS or who received nintedanib for ≤28 days in the DDI study ("initiated nintedanib" group). Analyses were descriptive. Analyses of FVC were based on observed data available at the respective time-point.
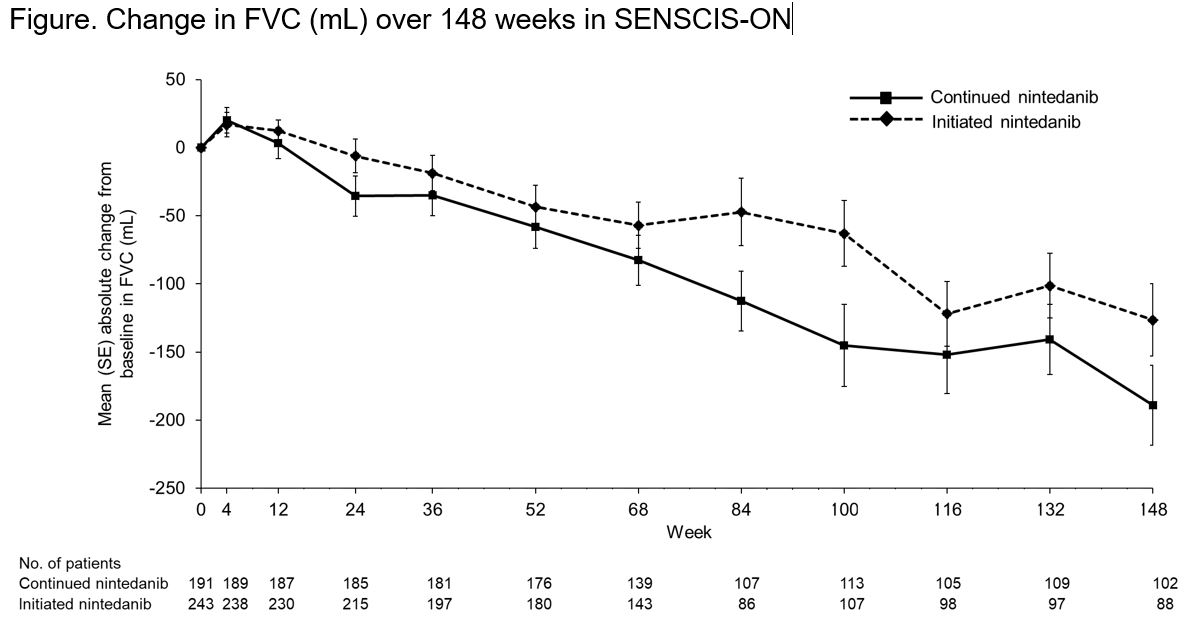
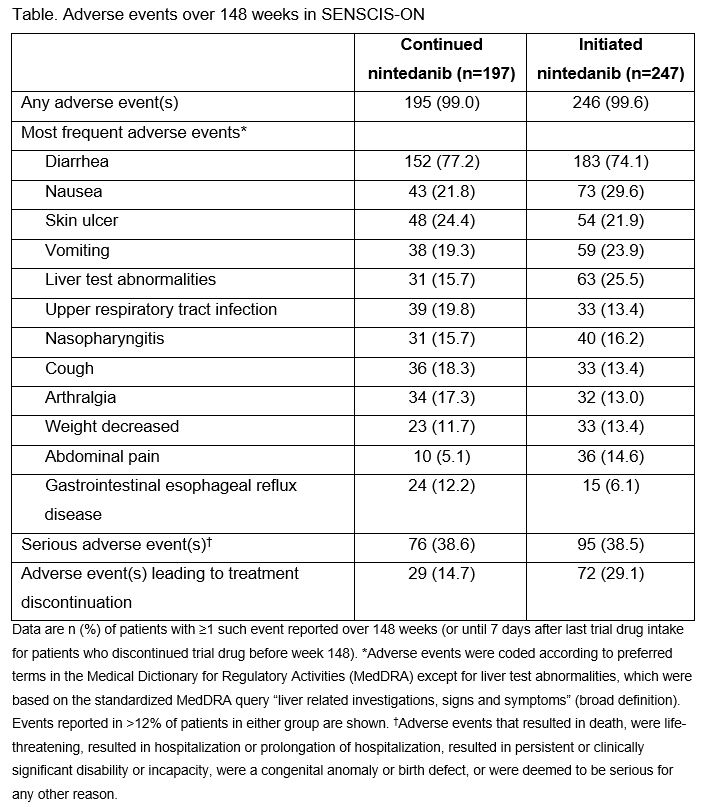
Results: The continued nintedanib group comprised 197 patients and the initiated nintedanib group comprised 247 patients (231 from SENSCIS, 16 from the DDI study). In these groups, respectively, mean (SD) FVC at the start of SENSCIS-ON was 2379 (754) mL and 70.4 (18.1) % predicted and 2443 (814) mL and 70.8 (17.9) % predicted. In total, 126 (64.0%) and 125 (50.6%) patients in the continued nintedanib and initiated nintedanib groups, respectively, were still receiving nintedanib at week 148 of SENSCIS-ON. Diarrhea was the most frequent adverse event (Table). Serious adverse events were reported in 76 (38.6%) patients in the continued nintedanib group and 95 (38.5%) patients in the initiated nintedanib group. Among patients who continued nintedanib and initiated nintedanib, respectively, 53 (26.9%) and 148 (59.9%) had ≥1 dose reduction and 72 (36.5%) and 131 (53.0%) had ≥1 treatment interruption. Adverse events led to discontinuation of nintedanib in 29 (14.7%) patients in the continued nintedanib group and 72 (29.1%) patients in the initiated nintedanib group. Mean (SE) changes in FVC from baseline to week 148 of SENSCIS-ON were −189.1 (29.5) mL in the continued nintedanib group and −126.4 (26.4) mL in the initiated nintedanib group (Figure).
Conclusion: The safety profile of nintedanib over 148 weeks of SENSCIS-ON was consistent with that reported in the SENSCIS trial, characterized mainly by gastrointestinal events that were manageable for most patients. These results support the potential use of nintedanib in the long-term treatment of patients with SSc-ILD.
Disclosures: Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca; M. Vonk, Boehringer Ingelheim, Ferrer, Galapagos, Janssen, Merck/MSD, Corbus, EUSTAR; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Azuma, Boehringer Ingelheim, Toray, Kyorin Pharma, Taiho; M. Mayes, Actelion Pharma, Mitsubishi-Tanabe, Boehringer Ingelheim, EICOS, Horizon Pharma, Prometheus, Corbus, Medtelligence; A. James, Boehringer Ingelheim - Elderbrook solutions; V. Kohlbrenner, Boehringer Ingelheim; M. Alves, Boehringer Ingelheim; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; K. Highland, Boehringer Ingelheim, Scleroderma Foundation.
Background/Purpose: In the randomized placebo-controlled SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) (mL/year) over 52 weeks by 44%, with adverse events that were manageable for most patients. SENSCIS-ON is an open-label extension study investigating adverse events and decline in FVC in patients with SSc-ILD treated with nintedanib over the longer term.
Methods: In the SENSCIS trial, patients received nintedanib or placebo until the last patient reached week 52 but for ≤100 weeks. Patients with SSc-ILD who completed the SENSCIS trial or a drug–drug interaction (DDI) study of nintedanib and oral contraceptive in which female patients with SSc-ILD received nintedanib for ≥14 days to approximately 28 days were eligible to enter SENSCIS-ON. We analyzed adverse events and changes from baseline in FVC (mL) over 148 weeks in patients who received nintedanib in SENSCIS and continued nintedanib in SENSCIS-ON ("continued nintedanib" group) and in patients who received placebo in SENSCIS or who received nintedanib for ≤28 days in the DDI study ("initiated nintedanib" group). Analyses were descriptive. Analyses of FVC were based on observed data available at the respective time-point.
Results: The continued nintedanib group comprised 197 patients and the initiated nintedanib group comprised 247 patients (231 from SENSCIS, 16 from the DDI study). In these groups, respectively, mean (SD) FVC at the start of SENSCIS-ON was 2379 (754) mL and 70.4 (18.1) % predicted and 2443 (814) mL and 70.8 (17.9) % predicted. In total, 126 (64.0%) and 125 (50.6%) patients in the continued nintedanib and initiated nintedanib groups, respectively, were still receiving nintedanib at week 148 of SENSCIS-ON. Diarrhea was the most frequent adverse event (Table). Serious adverse events were reported in 76 (38.6%) patients in the continued nintedanib group and 95 (38.5%) patients in the initiated nintedanib group. Among patients who continued nintedanib and initiated nintedanib, respectively, 53 (26.9%) and 148 (59.9%) had ≥1 dose reduction and 72 (36.5%) and 131 (53.0%) had ≥1 treatment interruption. Adverse events led to discontinuation of nintedanib in 29 (14.7%) patients in the continued nintedanib group and 72 (29.1%) patients in the initiated nintedanib group. Mean (SE) changes in FVC from baseline to week 148 of SENSCIS-ON were −189.1 (29.5) mL in the continued nintedanib group and −126.4 (26.4) mL in the initiated nintedanib group (Figure).
Conclusion: The safety profile of nintedanib over 148 weeks of SENSCIS-ON was consistent with that reported in the SENSCIS trial, characterized mainly by gastrointestinal events that were manageable for most patients. These results support the potential use of nintedanib in the long-term treatment of patients with SSc-ILD.


Disclosures: Y. Allanore, Boehringer Ingelheim, Sanofi, Janssen, AbbVie/Abbott, Menarini, Curzion, Medsenic, Prometheus, AstraZeneca; M. Vonk, Boehringer Ingelheim, Ferrer, Galapagos, Janssen, Merck/MSD, Corbus, EUSTAR; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Azuma, Boehringer Ingelheim, Toray, Kyorin Pharma, Taiho; M. Mayes, Actelion Pharma, Mitsubishi-Tanabe, Boehringer Ingelheim, EICOS, Horizon Pharma, Prometheus, Corbus, Medtelligence; A. James, Boehringer Ingelheim - Elderbrook solutions; V. Kohlbrenner, Boehringer Ingelheim; M. Alves, Boehringer Ingelheim; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; K. Highland, Boehringer Ingelheim, Scleroderma Foundation.

