Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1526: Developing a Screening Tool for the Detection of Interstitial Lung Disease in Systemic Sclerosis: The ILD-RISC Risk Score
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- cb
cosimo bruni, MD, PhD
University of Zurich & University of Florence
Florence, Florence, Italy
Abstract Poster Presenter(s)
cosimo bruni1, Lorenzo Tofani1, Håvard Fretheim2, Sophie Liem3, Arthiha Velauthapillai4, Hilde Jenssen Bjørkekjær5, Imon Barua2, Ilaria Galetti6, Alexandru Garaiman7, Mike Becker7, Anna-Maria Hoffmann-Vold2, Jeska de Vries-Bouwstra8, Madelon Vonk9, Jörg Distler10, Marco Matucci-Cerinic1 and Oliver Distler7, 1University of Florence, Florence, Italy, 2Oslo University Hospital, Oslo, Norway, 3LUMC, Leiden, Netherlands, 4Radboud University Medical Center, Nijmegen, Nijmegen, Netherlands, 5Department of Rheumatology, Hospital of Southern Norway, Kristiansand, Norway, Kristiansand, Norway, 6Gruppo Italiano Lotta alla Sclerodermia (GILS), Milano, Italy, 7Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 8Leiden University Medical Center, Leiden, Netherlands, 9Department of Rheumatology, Radboud University Medical Center, Nijmegen, Netherlands, 10Friedrich-Alexander-University Erlangen-Nürnberg (FAU) and University Hospital Erlangen, Erlangen, Germany
Background/Purpose: High resolution computed tomography (HRCT) is the gold standard for the diagnosis of systemic sclerosis associated interstitial lung disease (SSc-ILD). Although there is agreement in performing HRCT as a screening test at SSc diagnosis, some physicians do not regularly perform baseline HRCTs in all patients (Bruni et al, Clin Exp Rheumatol, in press). In addition, it is unclear according to which criteria HRCTs should be repeated during the follow-up of baseline ILD negative patients. We aimed to develop a risk score for the presence of SSc-ILD (the ILD-RISC score), to guide physicians in ordering both baseline and follow-up HRCTs.
Methods: The steering board included six SSc-ILD experts, two research fellows and a patient research partner. Items for regression analysis were selected according to face validity, feasibility, scientific background, and personal experience using the nominal group technique. The prediction model for the presence of ILD was developed from baseline visits of SSc patients from the six referral centers using multivariable logistic regression with backward selection. Patients were randomly divided into derivation and validation cohorts consisting of 66% and 34% of patients respectively. Patients with missing data in the selected covariates and in the ILD status were excluded. After identifying a cut-off favoring sensitivity >85% from the ROC curve analysis, the derived ILD-RISC score was applied first in the validation cohort and then longitudinally in a cohort of SSc patients with negative baseline HRCT.
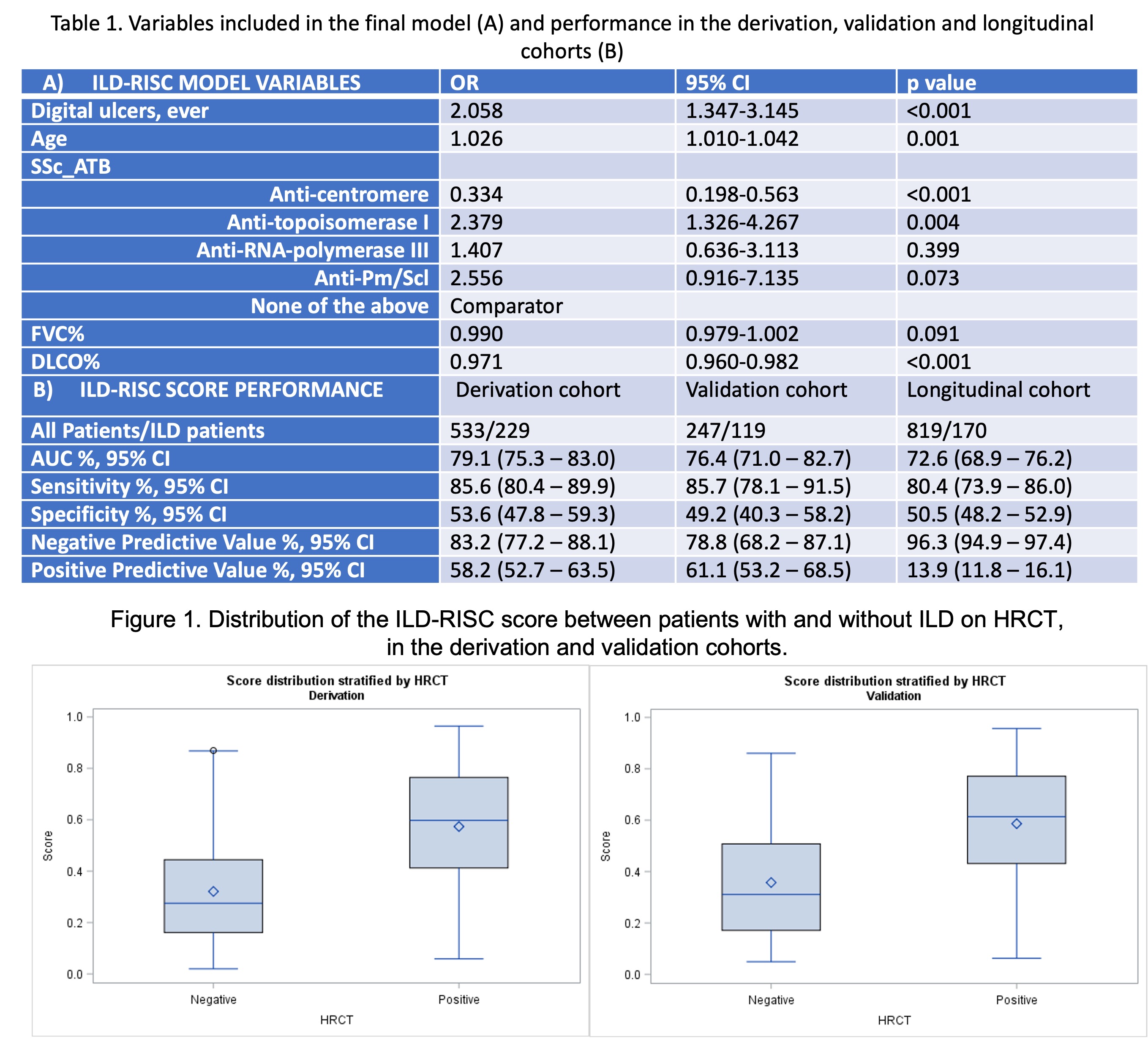
Results: The steering board selected 13 variables deemed important in the identification of SSc-ILD: sex, age, disease duration from first non-Raynaud's phenomenon symptom, skin subset, presence of esophageal symptoms, digital ulcers (DU) ever, arthritis ever, smoking ever, increased inflammatory markers, NYHA functional class, SSc autoantibodies positivity, FVC% and DLCO%. Among 780/3240 patients fulfilling the inclusion criteria, 533 (43% ILD) and 247 (48% ILD) respectively constituted the derivation and the validation cohort. In the derivation cohort, a model including FVC%, DLCO%, DU ever, age and SSc autoantibodies (Table 1A) showed an OR of 133.9 (95% CI 53.4-335.9) and an AUC of 79.1% (95% CI 75.3-83.0%) for the presence of ILD on HRCT (Figure 1). An ILD-RISC score >=0.3 showed sensitivity of 85.6% and specificity of 53.6%, which were replicated in the validation cohort (Table 1B). Among 819 patients with negative baseline HRCT, 170 (20.8%) developed ILD during a 3.8+/-3.0 years follow up (1988 visits). Longitudinally, the ILD-RISC score showed comparable sensitivity and specificity (Table 1B), resulting in almost 50% of visits (n=914/1809) in which the HRCT could be correctly skipped following an ILD-RISC score < 0.3.
Conclusion: We developed and validated the ILD-RISC score to predict the presence of ILD at time of diagnosis and evaluated its performance during follow-up. The ILD-RISC may be useful in routine practice when resources for HRCTs might be limited. Most importantly, it may help to decide when to order HRCTs at follow up, thus limiting unnecessary HRCTs and reducing the burden for patients and institutions.
Disclosures: c. bruni, Boehringer-Ingelheim, Eli Lilly; L. Tofani, None; H. Fretheim, Bayer, GSK and Actelion; S. Liem, None; A. Velauthapillai, None; H. Bjørkekjær, None; I. Barua, None; I. Galetti, None; A. Garaiman, None; M. Becker, Amgen, Bayer, Mepha, Novartis, Vifor, Novartis Foundation; A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche; J. de Vries-Bouwstra, None; M. Vonk, Boehringer Ingelheim, Ferrer, Galapagos, Janssen, Merck/MSD, Corbus, EUSTAR; J. Distler, Fibrocure, 4D Science; M. Matucci-Cerinic, Biogen, Eli Lilly, Chemomab, Sandoz, Merck/MSD, Pfizer, Behring, Janssen; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation.
Background/Purpose: High resolution computed tomography (HRCT) is the gold standard for the diagnosis of systemic sclerosis associated interstitial lung disease (SSc-ILD). Although there is agreement in performing HRCT as a screening test at SSc diagnosis, some physicians do not regularly perform baseline HRCTs in all patients (Bruni et al, Clin Exp Rheumatol, in press). In addition, it is unclear according to which criteria HRCTs should be repeated during the follow-up of baseline ILD negative patients. We aimed to develop a risk score for the presence of SSc-ILD (the ILD-RISC score), to guide physicians in ordering both baseline and follow-up HRCTs.
Methods: The steering board included six SSc-ILD experts, two research fellows and a patient research partner. Items for regression analysis were selected according to face validity, feasibility, scientific background, and personal experience using the nominal group technique. The prediction model for the presence of ILD was developed from baseline visits of SSc patients from the six referral centers using multivariable logistic regression with backward selection. Patients were randomly divided into derivation and validation cohorts consisting of 66% and 34% of patients respectively. Patients with missing data in the selected covariates and in the ILD status were excluded. After identifying a cut-off favoring sensitivity >85% from the ROC curve analysis, the derived ILD-RISC score was applied first in the validation cohort and then longitudinally in a cohort of SSc patients with negative baseline HRCT.
Results: The steering board selected 13 variables deemed important in the identification of SSc-ILD: sex, age, disease duration from first non-Raynaud's phenomenon symptom, skin subset, presence of esophageal symptoms, digital ulcers (DU) ever, arthritis ever, smoking ever, increased inflammatory markers, NYHA functional class, SSc autoantibodies positivity, FVC% and DLCO%. Among 780/3240 patients fulfilling the inclusion criteria, 533 (43% ILD) and 247 (48% ILD) respectively constituted the derivation and the validation cohort. In the derivation cohort, a model including FVC%, DLCO%, DU ever, age and SSc autoantibodies (Table 1A) showed an OR of 133.9 (95% CI 53.4-335.9) and an AUC of 79.1% (95% CI 75.3-83.0%) for the presence of ILD on HRCT (Figure 1). An ILD-RISC score >=0.3 showed sensitivity of 85.6% and specificity of 53.6%, which were replicated in the validation cohort (Table 1B). Among 819 patients with negative baseline HRCT, 170 (20.8%) developed ILD during a 3.8+/-3.0 years follow up (1988 visits). Longitudinally, the ILD-RISC score showed comparable sensitivity and specificity (Table 1B), resulting in almost 50% of visits (n=914/1809) in which the HRCT could be correctly skipped following an ILD-RISC score < 0.3.
Conclusion: We developed and validated the ILD-RISC score to predict the presence of ILD at time of diagnosis and evaluated its performance during follow-up. The ILD-RISC may be useful in routine practice when resources for HRCTs might be limited. Most importantly, it may help to decide when to order HRCTs at follow up, thus limiting unnecessary HRCTs and reducing the burden for patients and institutions.

Disclosures: c. bruni, Boehringer-Ingelheim, Eli Lilly; L. Tofani, None; H. Fretheim, Bayer, GSK and Actelion; S. Liem, None; A. Velauthapillai, None; H. Bjørkekjær, None; I. Barua, None; I. Galetti, None; A. Garaiman, None; M. Becker, Amgen, Bayer, Mepha, Novartis, Vifor, Novartis Foundation; A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche; J. de Vries-Bouwstra, None; M. Vonk, Boehringer Ingelheim, Ferrer, Galapagos, Janssen, Merck/MSD, Corbus, EUSTAR; J. Distler, Fibrocure, 4D Science; M. Matucci-Cerinic, Biogen, Eli Lilly, Chemomab, Sandoz, Merck/MSD, Pfizer, Behring, Janssen; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation.

