Back
Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes II: Complications (1591–1596)
1594: Incidence of Pneumocystis Jirovecii Pneumonia and Prophylaxis-Associated Adverse Events Among Patients with Systemic Lupus Erythematosus
Sunday, November 13, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 204
- YJ
Yiran Jiang, MD
Medical College of Wisconsin
Milwaukee, WI, United States
Presenting Author(s)
Yiran Jiang1, Ali Duarte-Garcia2, Mike Putman3 and David Gazeley4, 1Medical College of Wisconsin, Milwaukee, WI, 2Mayo Clinic, Rochester, MN, 3The Medical College of Wisconsin, Milwaukee, WI, 4Medical College of Wisconsin, Wauwatosa, WI
Background/Purpose: Pneumocystis jirovecii Pneumonia (PJP) is an opportunistic infection that may affect patients receiving immunosuppression. There are no consensus guidelines for PJP prophylaxis among patients with systemic lupus erythematosus (SLE). The objective of this study was to use an electronic health records (EHR) database to examine the incidence of PJP and prophylaxis-related adverse events among patients with SLE.
Methods: We performed a retrospective cohort analysis utilizing TriNetX, a federated database of de-identified records from 86 million patients across 54 organizations within the United States. Patients were required to have ≥3 ICD9/ICD10 SLE codes separated by at least 30 days and to have initiated therapy with mycophenolate mofetil (MMF) or cyclophosphamide. The index date was defined as the first administration of MMF or cyclophosphamide after the first ICD code. The observation period was limited to 6 months from the index date. PJP was defined by any hospitalization with primary or secondary diagnosis by ICD9/ICD10 code, any PJP code followed by treatment, or a positive PJP test. Patients who received PJP prophylaxis were propensity score matched (1:1) to patients who did not by the following variables: age, gender, race/ethnicity, regional location, total admissions in the prior year, and comorbidities including obesity, smoking status, congestive heart failure, chronic obstructive pulmonary disease, diabetes, liver disease, and cardiovascular disease. Incident rate ratios (IRR) for PJP and for adverse events commonly associated with PJP prophylaxis were estimated.
Results: A total of 4902 patients with SLE were identified. The mean age at the index date was 43.4 years, 86.4% were female, and the racial/ethnic make-up was 39.9% Black, 36.1% White, 13% Hispanic, and 2.8% Asian. The most common PJP prophylaxis was trimethoprim/sulfamethoxazole (942, 19.2%) out of 1385 patients who were prescribed prophylaxis. One PJP case was identified over 2282 person-years of follow-up for an overall incidence rate of 0.44 per 1000 person-years. The individual was a 49-year-old White female with renal disease who did not receive prophylaxis. She was diagnosed with PJP 132 days after receiving MMF and ultimately survived.
A total of 3664 patients were matched (1238 treated, 1238 controls), but the IRR for PJP could not be calculated due to insufficient cases of PJP. Patients who received prophylaxis had a higher rate of neutropenia (IRR 4.7, 95% confidence interval (95CI) 2.6-8.5), leukopenia (IRR 4.0, 95CI 2.6-6.0), Clostridium difficile infection (IRR 3.9, 95CI 1.4-10.3), hyperkalemia (IRR 3.7, 95CI 2.5-5.5), liver disease (IRR 2.0, 95CI 1.0-3.7), and thrombocytopenia (IRR 1.7, 95CI 1.1-2.4).
Conclusion: In a large EHR database study, only 1 case of PJP was observed. Prophylaxis against PJP was associated with a significantly higher incidence of adverse events. These data do not support universal prophylaxis against PJP. Future studies should investigate models for risk stratification.
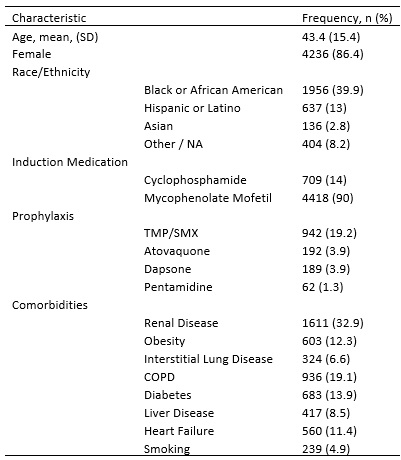 Table 1. Characteristics of Cohort of SLE Patients (n = 4902)
Table 1. Characteristics of Cohort of SLE Patients (n = 4902)
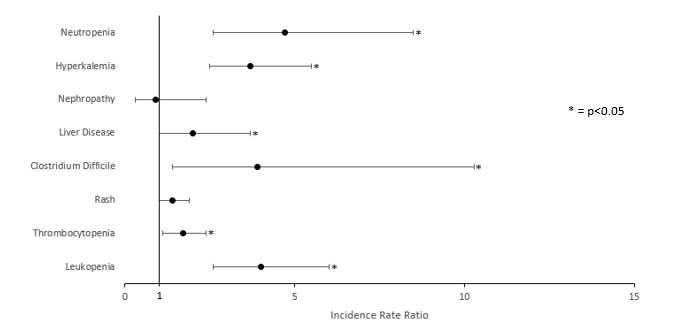 Figure 1. Comparison of Adverse Events in Patients With and Without PJP Prophylaxis
Figure 1. Comparison of Adverse Events in Patients With and Without PJP Prophylaxis
Disclosures: Y. Jiang, None; A. Duarte-Garcia, None; M. Putman, AstraZeneca, AbbVie/Abbott, Novartis; D. Gazeley, None.
Background/Purpose: Pneumocystis jirovecii Pneumonia (PJP) is an opportunistic infection that may affect patients receiving immunosuppression. There are no consensus guidelines for PJP prophylaxis among patients with systemic lupus erythematosus (SLE). The objective of this study was to use an electronic health records (EHR) database to examine the incidence of PJP and prophylaxis-related adverse events among patients with SLE.
Methods: We performed a retrospective cohort analysis utilizing TriNetX, a federated database of de-identified records from 86 million patients across 54 organizations within the United States. Patients were required to have ≥3 ICD9/ICD10 SLE codes separated by at least 30 days and to have initiated therapy with mycophenolate mofetil (MMF) or cyclophosphamide. The index date was defined as the first administration of MMF or cyclophosphamide after the first ICD code. The observation period was limited to 6 months from the index date. PJP was defined by any hospitalization with primary or secondary diagnosis by ICD9/ICD10 code, any PJP code followed by treatment, or a positive PJP test. Patients who received PJP prophylaxis were propensity score matched (1:1) to patients who did not by the following variables: age, gender, race/ethnicity, regional location, total admissions in the prior year, and comorbidities including obesity, smoking status, congestive heart failure, chronic obstructive pulmonary disease, diabetes, liver disease, and cardiovascular disease. Incident rate ratios (IRR) for PJP and for adverse events commonly associated with PJP prophylaxis were estimated.
Results: A total of 4902 patients with SLE were identified. The mean age at the index date was 43.4 years, 86.4% were female, and the racial/ethnic make-up was 39.9% Black, 36.1% White, 13% Hispanic, and 2.8% Asian. The most common PJP prophylaxis was trimethoprim/sulfamethoxazole (942, 19.2%) out of 1385 patients who were prescribed prophylaxis. One PJP case was identified over 2282 person-years of follow-up for an overall incidence rate of 0.44 per 1000 person-years. The individual was a 49-year-old White female with renal disease who did not receive prophylaxis. She was diagnosed with PJP 132 days after receiving MMF and ultimately survived.
A total of 3664 patients were matched (1238 treated, 1238 controls), but the IRR for PJP could not be calculated due to insufficient cases of PJP. Patients who received prophylaxis had a higher rate of neutropenia (IRR 4.7, 95% confidence interval (95CI) 2.6-8.5), leukopenia (IRR 4.0, 95CI 2.6-6.0), Clostridium difficile infection (IRR 3.9, 95CI 1.4-10.3), hyperkalemia (IRR 3.7, 95CI 2.5-5.5), liver disease (IRR 2.0, 95CI 1.0-3.7), and thrombocytopenia (IRR 1.7, 95CI 1.1-2.4).
Conclusion: In a large EHR database study, only 1 case of PJP was observed. Prophylaxis against PJP was associated with a significantly higher incidence of adverse events. These data do not support universal prophylaxis against PJP. Future studies should investigate models for risk stratification.
 Table 1. Characteristics of Cohort of SLE Patients (n = 4902)
Table 1. Characteristics of Cohort of SLE Patients (n = 4902) Figure 1. Comparison of Adverse Events in Patients With and Without PJP Prophylaxis
Figure 1. Comparison of Adverse Events in Patients With and Without PJP ProphylaxisDisclosures: Y. Jiang, None; A. Duarte-Garcia, None; M. Putman, AstraZeneca, AbbVie/Abbott, Novartis; D. Gazeley, None.

