Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatment II: Pre- and Early Disease (1603–1607)
1606: An Expanded Anti-citrullinated Protein Antibody Profile Derived Using Unsupervised Machine Learning Predicts Treatment Responses to Biologic Therapies in Rheumatoid Arthritis
Sunday, November 13, 2022
5:15 PM – 5:25 PM Eastern Time
Location: Room 121
.jpg)
Nozima Aripova, BS
University of Nebraska Medical Center
Omaha, NE, United States
Presenting Author(s)
nozima Aripova1, George Reed2, Bryant England1, William Robinson3, Dimitrios Pappas4, Joel Kremer5, Geoffrey Thiele1 and Ted Mikuls6, 1University of Nebraska Medical Center, Omaha, NE, 2The Corrona Research Foundation and University of Massachusetts, Albany, NY, 3Stanford University School of Medicine, Palo Alto, CA, 4CorEvitas, LLC, Waltham, MA, 5The Corrona Research Foundation, Delray Beach, FL, 6Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Rheumatoid arthritis (RA) treatments have advanced with the availability of biologic therapies. Despite these advances, 30-40% of patients receiving a biologic do not adequately respond to treatment. In addition to being highly specific and potentially pathogenic in RA, anti-cyclic citrullinated peptide (CCP) antibody positivity has been preliminarily associated with biologic treatment responses in RA. Recognizing that the commercially available anti-CCP assay represents a 'global' anti-citrullinated protein antibody (ACPA) measure, it is possible that antibodies to specific citrullinated peptides/proteins or distinct ACPA patterns could yield greater predictive capacity. The goal of this study was to evaluate whether an expanded ACPA profile predicts treatment response among patients with RA initiating biologic therapy.
Methods: The study included banked serum and data from Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions (CERTAIN). Participants were categorized by treatment: 1) biologic-naïve initiating anti-TNF (n=386), 2) biologic-exposed initiating non-TNF/non-abatacept (n=627), and 3) biologic naïve initiating abatacept (n=79). ACPAs to 25 citrullinated peptides were measured using a multiplex bead-based assay and banked enrollment serum. Values were log-transformed and standardized. Principal components analysis, an unsupervised machine learning approach, was performed, retaining components with eigenvalues >1. Associations of principal component (PC) scores (in quartiles) and commercially tested anti-CCP antibody (≤15, 16-250 or >250 U/ml) with EULAR (good/moderate/none) treatment response at 6 months was examined using simple and fully-adjusted ordinal regression models. The assumption of proportional odds was tested using a Brant test.
Results: Participants (n=1092) had a mean age of 57.1 (SD:12.8) years; 79.4% were women. At 6 months, 68.5% achieved a moderate or good EULAR response. PC analysis revealed the presence of 3 ACPA PCs with an eigenvalue >1, cumulatively explaining 70.2% of the overall ACPA variation. In a simple model including the 3 PCs and anti-CCP antibody, only PC1 and PC2 were associated with treatment response; PC3 and anti-CCP were excluded from further analyses. Loading values of each ACPA for PC1 and PC2 are shown in Figure 1. All ACPA yielded similar loading values for PC1 while individual ACPA demonstrated both positive and negative loadings for PC2. Multivariable associations of PC1 and PC2 with EULAR response following full adjustment are shown in Figure 2. The highest quartile for PC1 (OR 1.76; 95% CI 1.22-2.53) and PC2 (OR 1.74; 95% CI 1.23-2.46) were independently associated with treatment response (Figure 2).
Conclusion: These findings demonstrate that an expanded ACPA profile may be used to predict biologic treatment responses in RA and appears to be predictive independent of commercially available anti-CCP antibody testing. Further study is needed to identify other factors and/or biomarkers that could enhance this predictive capacity to effectively prioritize between different biologic therapies in RA.
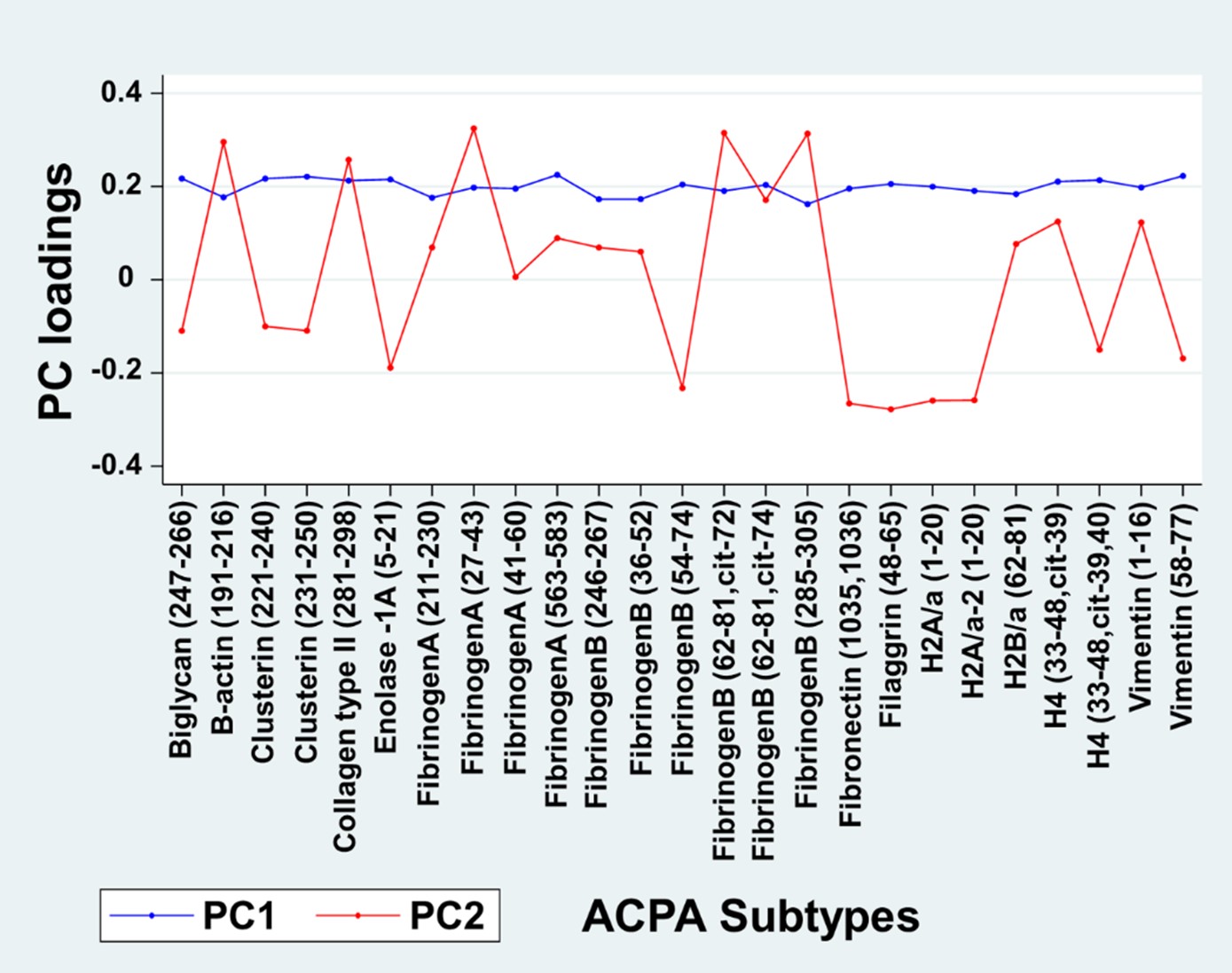 Figure 1. Loading Scores for ACPA Subtypes for PC1 and PC2 (n=1092). Individual ACPA subtype values were reduced into two principal components (PC). PC1 and PC2 represent 64% of cumulative variation of all ACPA values. PC3 demonstrated no association with treatments response and was excluded from further analyses. The loading scores for each component were tested for association with EULAR response using ordinal logistic regression.
Figure 1. Loading Scores for ACPA Subtypes for PC1 and PC2 (n=1092). Individual ACPA subtype values were reduced into two principal components (PC). PC1 and PC2 represent 64% of cumulative variation of all ACPA values. PC3 demonstrated no association with treatments response and was excluded from further analyses. The loading scores for each component were tested for association with EULAR response using ordinal logistic regression.
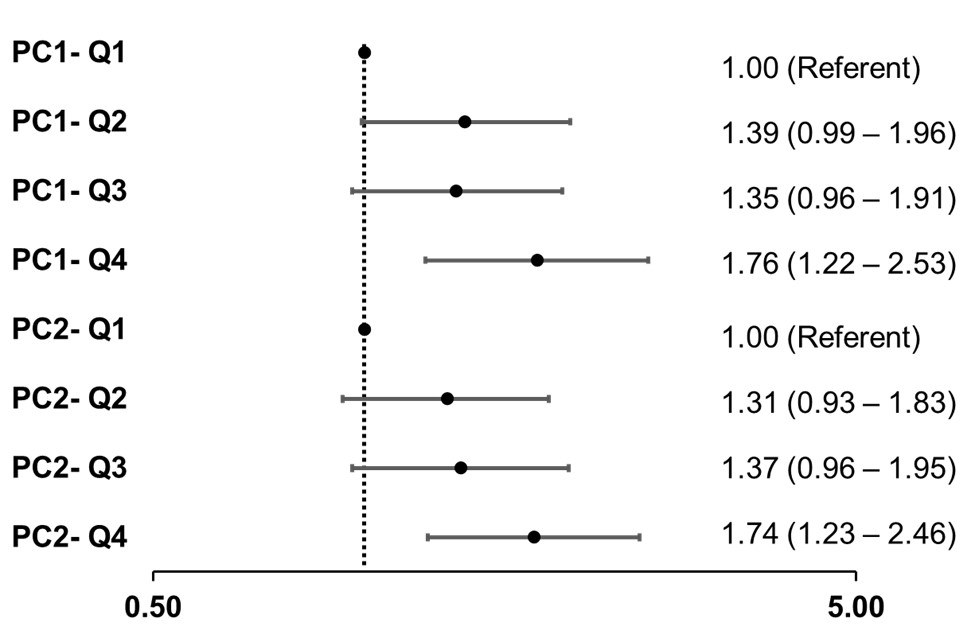 Figure 2. Associations of ACPA Subtypes PC1 and PC2 with EULAR Response (n=1092). Multivariable adjusted models developed using ordinal logistic regression. Q-quartile; PC, principal component. Results are shown from single model including PC1, PC2, and the following covariates: sex, age, race, BMI, smoking status, disease duration, baseline disease activity, diabetes, cardiovascular disease, prednisone, methotrexate, and treatment assignment.
Figure 2. Associations of ACPA Subtypes PC1 and PC2 with EULAR Response (n=1092). Multivariable adjusted models developed using ordinal logistic regression. Q-quartile; PC, principal component. Results are shown from single model including PC1, PC2, and the following covariates: sex, age, race, BMI, smoking status, disease duration, baseline disease activity, diabetes, cardiovascular disease, prednisone, methotrexate, and treatment assignment.
Disclosures: n. Aripova, None; G. Reed, CorEvitas, Corrona Research Foundation; B. England, Boehringer-Ingelheim; W. Robinson, None; D. Pappas, CorEvitas, Novartis, Sanofi, Genentech, Roche, AbbVie; J. Kremer, CorEvitas; G. Thiele, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.
Background/Purpose: Rheumatoid arthritis (RA) treatments have advanced with the availability of biologic therapies. Despite these advances, 30-40% of patients receiving a biologic do not adequately respond to treatment. In addition to being highly specific and potentially pathogenic in RA, anti-cyclic citrullinated peptide (CCP) antibody positivity has been preliminarily associated with biologic treatment responses in RA. Recognizing that the commercially available anti-CCP assay represents a 'global' anti-citrullinated protein antibody (ACPA) measure, it is possible that antibodies to specific citrullinated peptides/proteins or distinct ACPA patterns could yield greater predictive capacity. The goal of this study was to evaluate whether an expanded ACPA profile predicts treatment response among patients with RA initiating biologic therapy.
Methods: The study included banked serum and data from Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions (CERTAIN). Participants were categorized by treatment: 1) biologic-naïve initiating anti-TNF (n=386), 2) biologic-exposed initiating non-TNF/non-abatacept (n=627), and 3) biologic naïve initiating abatacept (n=79). ACPAs to 25 citrullinated peptides were measured using a multiplex bead-based assay and banked enrollment serum. Values were log-transformed and standardized. Principal components analysis, an unsupervised machine learning approach, was performed, retaining components with eigenvalues >1. Associations of principal component (PC) scores (in quartiles) and commercially tested anti-CCP antibody (≤15, 16-250 or >250 U/ml) with EULAR (good/moderate/none) treatment response at 6 months was examined using simple and fully-adjusted ordinal regression models. The assumption of proportional odds was tested using a Brant test.
Results: Participants (n=1092) had a mean age of 57.1 (SD:12.8) years; 79.4% were women. At 6 months, 68.5% achieved a moderate or good EULAR response. PC analysis revealed the presence of 3 ACPA PCs with an eigenvalue >1, cumulatively explaining 70.2% of the overall ACPA variation. In a simple model including the 3 PCs and anti-CCP antibody, only PC1 and PC2 were associated with treatment response; PC3 and anti-CCP were excluded from further analyses. Loading values of each ACPA for PC1 and PC2 are shown in Figure 1. All ACPA yielded similar loading values for PC1 while individual ACPA demonstrated both positive and negative loadings for PC2. Multivariable associations of PC1 and PC2 with EULAR response following full adjustment are shown in Figure 2. The highest quartile for PC1 (OR 1.76; 95% CI 1.22-2.53) and PC2 (OR 1.74; 95% CI 1.23-2.46) were independently associated with treatment response (Figure 2).
Conclusion: These findings demonstrate that an expanded ACPA profile may be used to predict biologic treatment responses in RA and appears to be predictive independent of commercially available anti-CCP antibody testing. Further study is needed to identify other factors and/or biomarkers that could enhance this predictive capacity to effectively prioritize between different biologic therapies in RA.
 Figure 1. Loading Scores for ACPA Subtypes for PC1 and PC2 (n=1092). Individual ACPA subtype values were reduced into two principal components (PC). PC1 and PC2 represent 64% of cumulative variation of all ACPA values. PC3 demonstrated no association with treatments response and was excluded from further analyses. The loading scores for each component were tested for association with EULAR response using ordinal logistic regression.
Figure 1. Loading Scores for ACPA Subtypes for PC1 and PC2 (n=1092). Individual ACPA subtype values were reduced into two principal components (PC). PC1 and PC2 represent 64% of cumulative variation of all ACPA values. PC3 demonstrated no association with treatments response and was excluded from further analyses. The loading scores for each component were tested for association with EULAR response using ordinal logistic regression. Figure 2. Associations of ACPA Subtypes PC1 and PC2 with EULAR Response (n=1092). Multivariable adjusted models developed using ordinal logistic regression. Q-quartile; PC, principal component. Results are shown from single model including PC1, PC2, and the following covariates: sex, age, race, BMI, smoking status, disease duration, baseline disease activity, diabetes, cardiovascular disease, prednisone, methotrexate, and treatment assignment.
Figure 2. Associations of ACPA Subtypes PC1 and PC2 with EULAR Response (n=1092). Multivariable adjusted models developed using ordinal logistic regression. Q-quartile; PC, principal component. Results are shown from single model including PC1, PC2, and the following covariates: sex, age, race, BMI, smoking status, disease duration, baseline disease activity, diabetes, cardiovascular disease, prednisone, methotrexate, and treatment assignment.Disclosures: n. Aripova, None; G. Reed, CorEvitas, Corrona Research Foundation; B. England, Boehringer-Ingelheim; W. Robinson, None; D. Pappas, CorEvitas, Novartis, Sanofi, Genentech, Roche, AbbVie; J. Kremer, CorEvitas; G. Thiele, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.

