Back
Abstract Session
Sjögren's syndrome
Session: Abstracts: Sjögren's Syndrome – Basic and Clinical Science (1625–1628)
1627: BAFF-var Is a New Predisposing Factor for Primary Sjögren’s Syndrome (pSS) and Impacts Disease Activity
Sunday, November 13, 2022
5:30 PM – 5:40 PM Eastern Time
Location: Room 204
- MD
Marie Dulin, MD
INSERM UMR1184
Paris, France
Presenting Author(s)
Marie Dulin1, Maxime Beydon2, Bineta Ly3, Veronique Le Guern4, Raphaèle Seror5, Xavier Mariette6 and Gaetane Nocturne7, 1Kremlin Bicêtre Hospital - APHP, Paris, France, 2Université Paris Cité, Paris, France, 3INSERM UMR1184, Le Kremlin Bicêtre, France, 4Hôpital Cochin, Paris, France, 5University Hospital Paris-Saclay, Le Kremlin Bicêtre, France, 6Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 7APHP, Le Kremlin Bicêtre, France
Background/Purpose: Chronic B cell activation plays a key role in pSS pathogeny. BAFF (B-cell activating factor) is largely involved in this process and positive results of therapies targeting this pathway highlight its involvement. BAFF serum level has shown to be increased in pSS patients and the reasons for this increase remain partially understood.
A functional polymorphism within TNFSF13B coding for BAFF, called BAFF-var, has been recently described. Its presence was not assessable in the GWAS studies. It leads to a shorter transcript that escapes microRNA inhibition resulting in increased BAFF level. BAFF-var has been shown to be more frequent in patients with lupus and multiple sclerosis. More recently, it has been shown to be associated with risk of lupus nephritis.
Our objective was to assess BAFF-var prevalence among pSS patients and to test the association of this variant with disease characteristics.
Methods: We conducted a retrospective and bicentric study on a French cohort from national referral center for Sjögren's disease. Patients who met the 2016 ACR/EULAR diagnostic criteria for pSS with available DNA or sera were included. We performed Taqman allelic discrimination assay on DNA samples for genotyping BAFF-var. Soluble BAFF was measured by enzyme-linked immunosorbent assay. Clinical and biological data were collected at diagnosis and during follow up. Disease activity was assessed by a cumulative ESSDAI score (EULAR Sjögren's Syndrome Disease Activity Index) which was calculated by considering for each domain the highest value reached by the patient during follow-up.
Results: We included 431 pSS patients. DNA and sera samples were available for 408 and 383 patients, respectively. Among the 408 patients tested, we found that 37 patients were BAFF-var carriers (36 heterozygotes and 1 homozygote), representing a variant prevalence of 9% in pSS population compared to a prevalence of 2% in general population (1000 Genomes Project). We found that BAFF-var was significantly associated with higher soluble BAFF level (1845 pg/ml vs 1335 pg/ml, Figure 1), Focusing on 222 patients with medical data collected, we found that BAFF-var was also significantly associated with a more active disease (Table 1), a more frequent lymph node involvement and an increased risk of Marginal Zone lymphoma (16,2% in patients with BAFF-var vs 4,9% in others, p= 0,0229). These results remained significant after adjusting for gender, ethnicity, age at diagnosis and length of follow-up. We observed a correlation between BAFF serum level and cumulative disease activity (r = 03881, p < 0,0001). In multivariate analysis, soluble BAFF level was the main driver of disease activity, independently of BAFF-var (Table 2).
Conclusion: We found an increased prevalence of BAFF-var in pSS compared with what is known in the European population. Genotyping of controls subjects from the same Paris area is on-going and will be presented at the ACR Convergence.
This variant, by increasing BAFF serum level, is associated with a particular phenotype of pSS. Even if BAFF-var is not the only driver of disease activity, it appears to be a predictive marker of disease severity, allowing a better stratification of patients and a more personalized management.
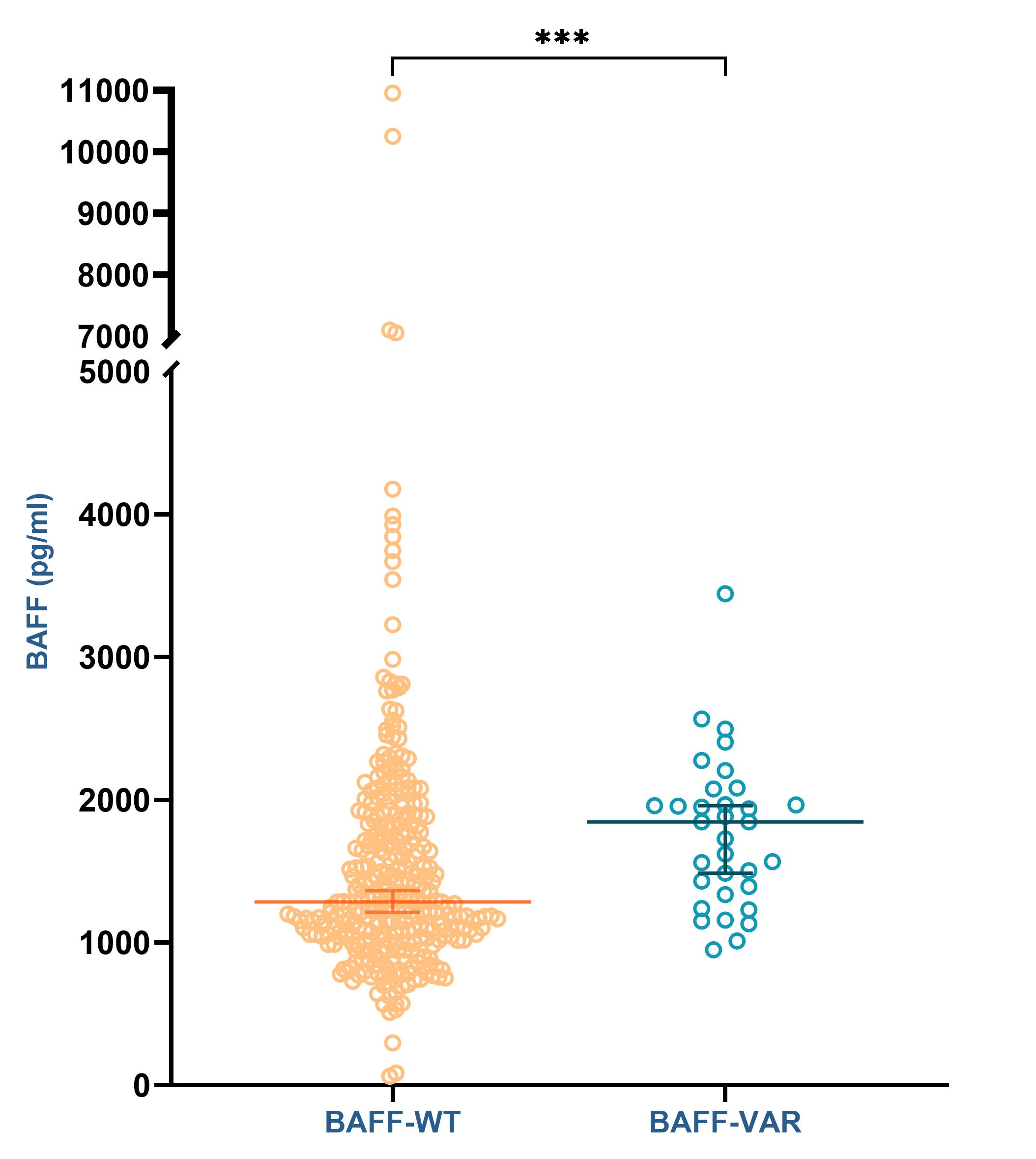 Figure 1: Soluble BAFF level according to BAFF-var genotype
Figure 1: Soluble BAFF level according to BAFF-var genotype
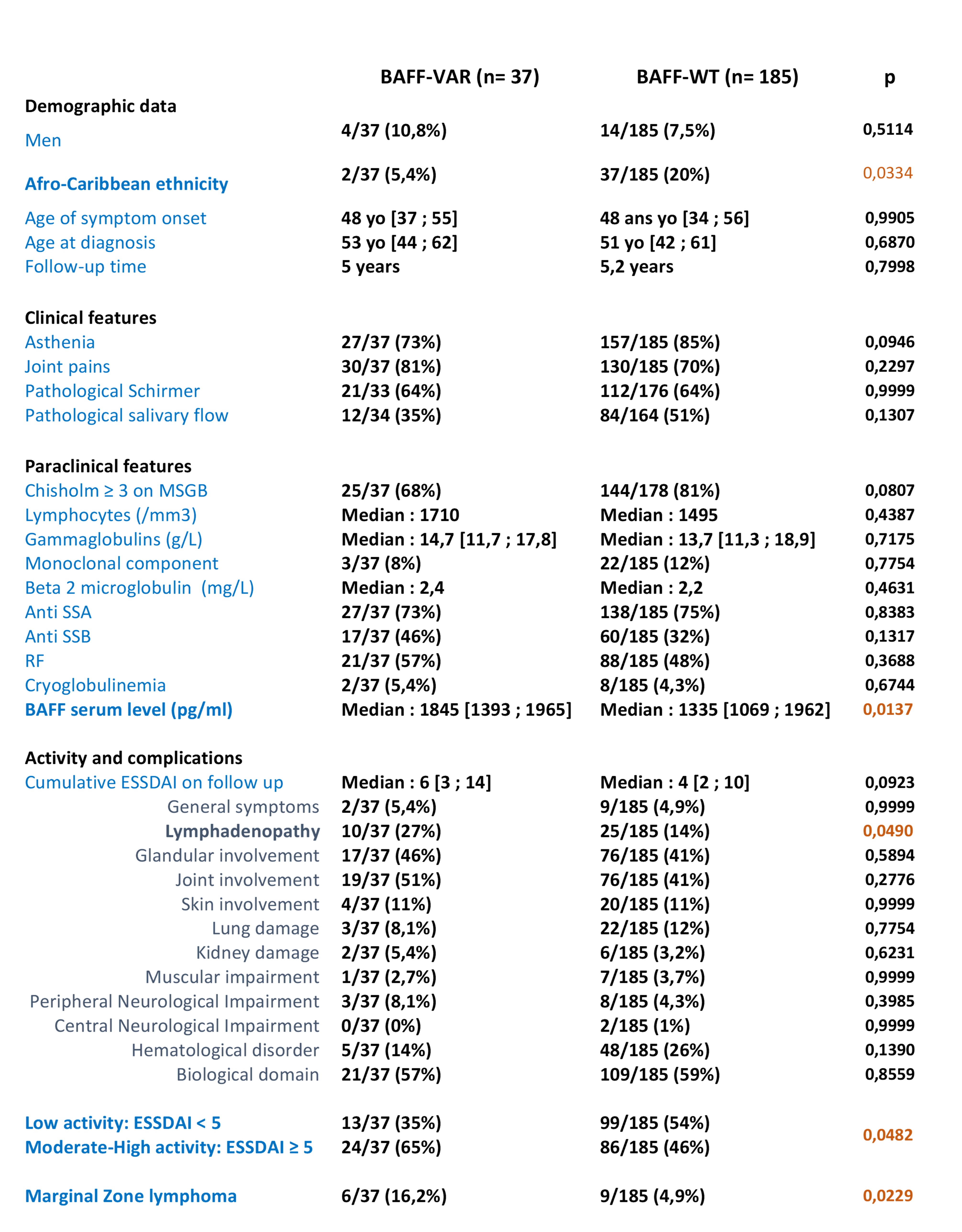 Table 1: Disease characteristics in pSS patients according to BAFF-var genotype (results are presented as number (%) or median [1st and 3rd quartile])
Table 1: Disease characteristics in pSS patients according to BAFF-var genotype (results are presented as number (%) or median [1st and 3rd quartile])
.jpg) Table 2: Multivariate analysis of characteristics associated with cumulative disease activity
Table 2: Multivariate analysis of characteristics associated with cumulative disease activity
Disclosures: M. Dulin, None; M. Beydon, None; B. Ly, None; V. Le Guern, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.
Background/Purpose: Chronic B cell activation plays a key role in pSS pathogeny. BAFF (B-cell activating factor) is largely involved in this process and positive results of therapies targeting this pathway highlight its involvement. BAFF serum level has shown to be increased in pSS patients and the reasons for this increase remain partially understood.
A functional polymorphism within TNFSF13B coding for BAFF, called BAFF-var, has been recently described. Its presence was not assessable in the GWAS studies. It leads to a shorter transcript that escapes microRNA inhibition resulting in increased BAFF level. BAFF-var has been shown to be more frequent in patients with lupus and multiple sclerosis. More recently, it has been shown to be associated with risk of lupus nephritis.
Our objective was to assess BAFF-var prevalence among pSS patients and to test the association of this variant with disease characteristics.
Methods: We conducted a retrospective and bicentric study on a French cohort from national referral center for Sjögren's disease. Patients who met the 2016 ACR/EULAR diagnostic criteria for pSS with available DNA or sera were included. We performed Taqman allelic discrimination assay on DNA samples for genotyping BAFF-var. Soluble BAFF was measured by enzyme-linked immunosorbent assay. Clinical and biological data were collected at diagnosis and during follow up. Disease activity was assessed by a cumulative ESSDAI score (EULAR Sjögren's Syndrome Disease Activity Index) which was calculated by considering for each domain the highest value reached by the patient during follow-up.
Results: We included 431 pSS patients. DNA and sera samples were available for 408 and 383 patients, respectively. Among the 408 patients tested, we found that 37 patients were BAFF-var carriers (36 heterozygotes and 1 homozygote), representing a variant prevalence of 9% in pSS population compared to a prevalence of 2% in general population (1000 Genomes Project). We found that BAFF-var was significantly associated with higher soluble BAFF level (1845 pg/ml vs 1335 pg/ml, Figure 1), Focusing on 222 patients with medical data collected, we found that BAFF-var was also significantly associated with a more active disease (Table 1), a more frequent lymph node involvement and an increased risk of Marginal Zone lymphoma (16,2% in patients with BAFF-var vs 4,9% in others, p= 0,0229). These results remained significant after adjusting for gender, ethnicity, age at diagnosis and length of follow-up. We observed a correlation between BAFF serum level and cumulative disease activity (r = 03881, p < 0,0001). In multivariate analysis, soluble BAFF level was the main driver of disease activity, independently of BAFF-var (Table 2).
Conclusion: We found an increased prevalence of BAFF-var in pSS compared with what is known in the European population. Genotyping of controls subjects from the same Paris area is on-going and will be presented at the ACR Convergence.
This variant, by increasing BAFF serum level, is associated with a particular phenotype of pSS. Even if BAFF-var is not the only driver of disease activity, it appears to be a predictive marker of disease severity, allowing a better stratification of patients and a more personalized management.
 Figure 1: Soluble BAFF level according to BAFF-var genotype
Figure 1: Soluble BAFF level according to BAFF-var genotype Table 1: Disease characteristics in pSS patients according to BAFF-var genotype (results are presented as number (%) or median [1st and 3rd quartile])
Table 1: Disease characteristics in pSS patients according to BAFF-var genotype (results are presented as number (%) or median [1st and 3rd quartile]).jpg) Table 2: Multivariate analysis of characteristics associated with cumulative disease activity
Table 2: Multivariate analysis of characteristics associated with cumulative disease activityDisclosures: M. Dulin, None; M. Beydon, None; B. Ly, None; V. Le Guern, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.

