Back
Abstract Session
Session: Abstracts: Immunological Complications of Medical Therapy (1667–1670)
1669: Comparing the Safety and Effectiveness of Methotrexate, TNF and IL6 Inhibitors for the Treatment of Checkpoint Inhibitor Arthritis
Monday, November 14, 2022
11:00 AM – 11:10 AM Eastern Time
Location: Room 201
.jpg)
Anne Bass, MD
Hospital for Special Surgery, Weill Cornell Medicine
New York, NY, United States
Presenting Author(s)
Anne Bass1, Noha Abdel-Wahab2, Pankti Reid3, Jeffrey Sparks4, Cassandra Calabrese5, Deanna Jannat-Khah, DrPH, MSPH6, Diviya Rajesh6, Nilasha Ghosh6, Komal Mushtaq7, Farah Al Haj8, Adewunmi Falohun9, Lydia Gedmintas10, Lindsey MacFarlane11, Senada Arabelovic11, Adi Diab2, Ami Shah12, Clifton O. Bingham III13, Karmela Kim Chan6 and Laura C. Cappelli14, 1Hospital for Special Surgery/Weill Cornell Medicine, New York, NY, 2UT MD Anderson Cancer Center, Houston, TX, 3University of Chicago Medical Center, Chicago, IL, 4Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 5Cleveland Clinic Foundation, Cleveland Heights, OH, 6Hospital for Special Surgery, New York, NY, 7Cleveland Clinic, Cleveland, OH, 8Sinai Grace Hospital/ Detroit Medical Center, Detroit, MI, 9MD Anderson Cancer Center, Houston, TX, 10Brigham and Women's Hospital, Boston, MA, 11Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 12Johns Hopkins Rheumatology, Baltimore, MD, 13Johns Hopkins University, Baltimore, MD, 14Johns Hopkins School of Medicine, Baltimore, MD
Background/Purpose: Immune checkpoint inhibitor associated arthritis (ICI-A) affects ∼4% of ICI-treated cancer patients and often persists. Given its long duration, it is important to identify effective treatments that do not interfere with cancer responses. However, there are no studies comparing the safety or effectiveness of disease modifying anti-rheumatic drugs (DMARDs) for this condition.
Methods: This was a retrospective multicenter observational study. Inclusion criteria were 1. ICI-A or ICI-associated PMR with ≥ 1 inflamed peripheral joint and 2. Treatment with a TNF or IL6R inhibitor (TNFi or IL6Ri), and/or methotrexate (MTX). Patients with a preexisting autoimmune disease were excluded. The primary outcome was time to cancer progression from time of ICI initiation. The secondary outcome was time to arthritis control, defined as grade 1 arthritis (mild pain, not interfering with function) and prednisone ≤ 10 mg after DMARD initiation. Descriptive statistics and comparisons were performed using Fisher's exact test, T-Tests, Wilcoxon rank sum tests and ANOVA as appropriate. Cox proportional hazard models were constructed to identify factors associated with cancer progression and arthritis control. Variables included in the models were DMARD (exposure of interest), ICI and cancer type, age, sex, and time from ICI to DMARD initiation.
Results: One hundred twenty-two patients were included, mean (SD) age 62 (12) years, 39% female, 39% with melanoma, 8% non-small cell lung cancer, 19% renal cell carcinoma and 7% bladder cancer (Table 1). Seventy percent received PD(L)1 monotherapy. Median (IQR) time from ICI initiation to ICI-A onset was 170 (63,369) days, and from ICI-A onset to initiation of a DMARD 385 (258,668) days. Time from ICI initiation to DMARD start was shortest for IL6Ri-only treated patients. Median CDAI at rheumatology presentation was 22 (11,26) and 41% of the patients had other concomitant immune related adverse events. ICI were held for ICI-A in 21% of patients and discontinued in 41%. The DMARD used was a TNFi in 13%, IL6Ri 35%, MTX 31%, TNFi/MTX 9% and IL6Ri/MTX 11%. Total duration of DMARD treatment was shortest for TNFi-only treated patients. A Kaplan-Meier curve showing time to cancer progression by DMARD treatment is shown in Figure 1, and time to arthritis control in Figure 2. Compared to patients treated with a TNFi ± MTX, patients treated with IL6Ri ± MTX had a similar time to cancer progression (HR 0.74; 95%CI 0.32-1.72, P=0.49) and to arthritis control (HR 0.84; 95%CI 0.46-1.50, P=0.55). Patients treated with MTX alone had a trend toward a longer time to cancer progression (HR 0.39; 95%CI 0.13-0.1.19, P=0.10) and a longer time to arthritis control (HR 0.37; 95%CI 0.19-0.72, P=0.004) than patients treated with a TNFi ± MTX.
Conclusion: This retrospective cohort study suggests that ICI-A patients treated with either a TNFi ± MTX or an IL6Ri ± MTX have a shorter time to cancer progression but faster arthritis control than patients treated with MTX alone. A prospective randomized controlled trial is needed to verify these findings and to identify the optimal approach to managing patients with high grade ICI-A.
.jpg) Table 1. Description of the study cohort
Table 1. Description of the study cohort
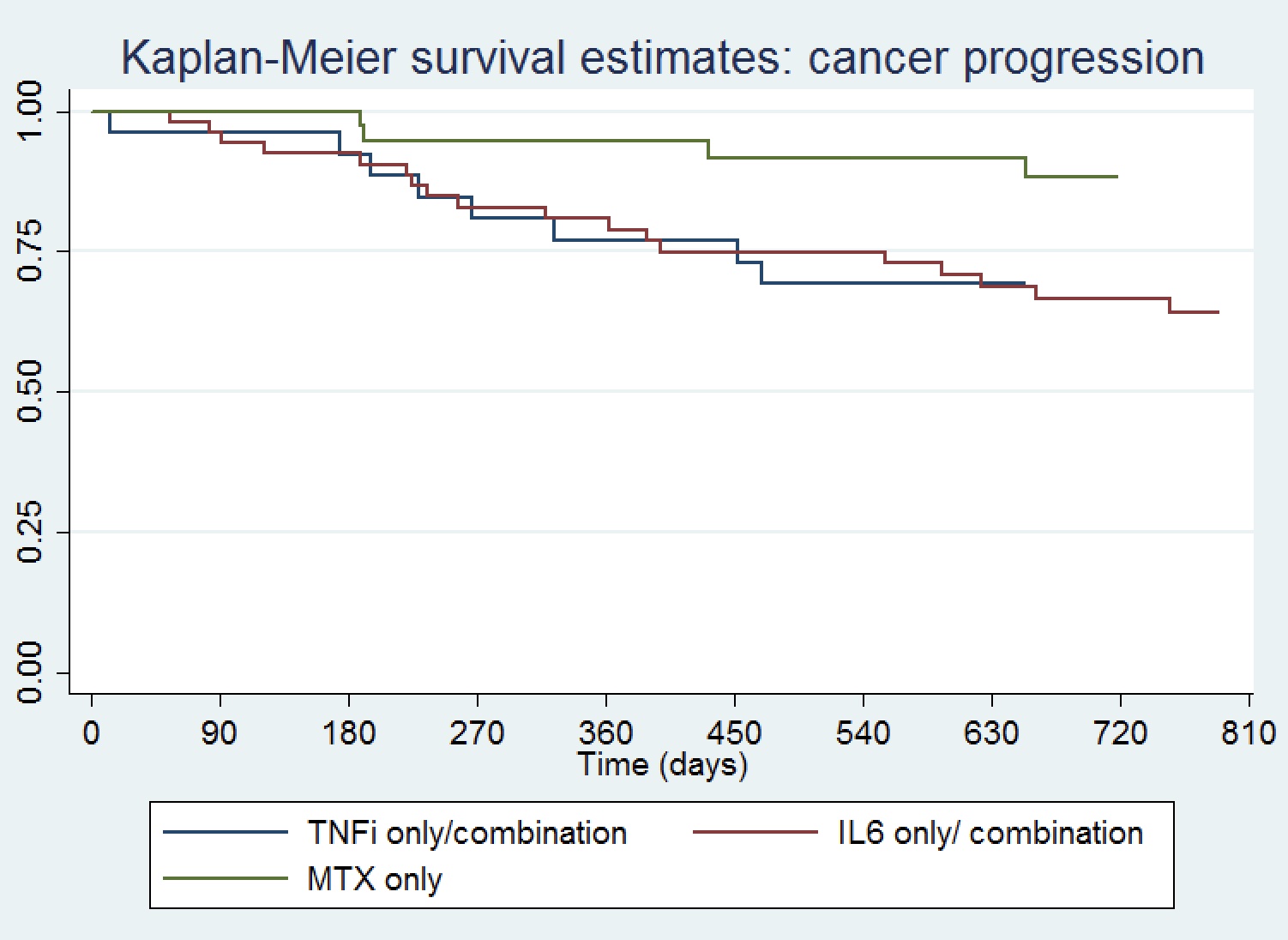 Figure 1. Kaplan-Meier curve showing time to cancer progression by DMARD treatment
Figure 1. Kaplan-Meier curve showing time to cancer progression by DMARD treatment
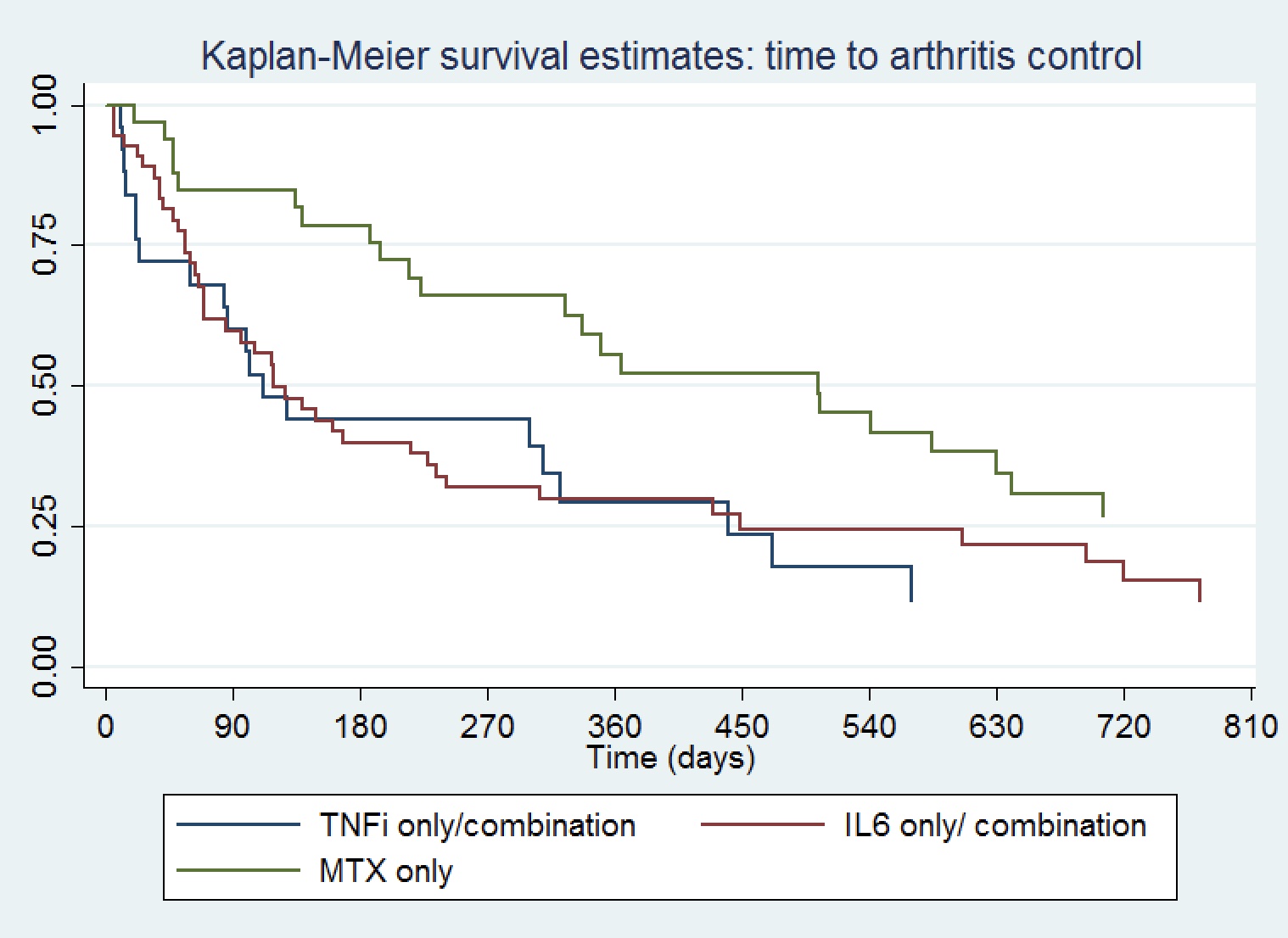 Figure 2. Kaplan-Meier curve showing time to arthritis control by DMARD treatment
Figure 2. Kaplan-Meier curve showing time to arthritis control by DMARD treatment
Disclosures: A. Bass, None; N. Abdel-Wahab, None; P. Reid, provisional patent; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; C. Calabrese, Sanofi, Astrazenica; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; D. Rajesh, None; N. Ghosh, GoodRx, Musculo; K. Mushtaq, None; F. Al Haj, None; A. Falohun, None; L. Gedmintas, None; L. MacFarlane, None; S. Arabelovic, None; A. Diab, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; K. Chan, None; L. C. Cappelli, Bristol-Myers Squibb(BMS).
Background/Purpose: Immune checkpoint inhibitor associated arthritis (ICI-A) affects ∼4% of ICI-treated cancer patients and often persists. Given its long duration, it is important to identify effective treatments that do not interfere with cancer responses. However, there are no studies comparing the safety or effectiveness of disease modifying anti-rheumatic drugs (DMARDs) for this condition.
Methods: This was a retrospective multicenter observational study. Inclusion criteria were 1. ICI-A or ICI-associated PMR with ≥ 1 inflamed peripheral joint and 2. Treatment with a TNF or IL6R inhibitor (TNFi or IL6Ri), and/or methotrexate (MTX). Patients with a preexisting autoimmune disease were excluded. The primary outcome was time to cancer progression from time of ICI initiation. The secondary outcome was time to arthritis control, defined as grade 1 arthritis (mild pain, not interfering with function) and prednisone ≤ 10 mg after DMARD initiation. Descriptive statistics and comparisons were performed using Fisher's exact test, T-Tests, Wilcoxon rank sum tests and ANOVA as appropriate. Cox proportional hazard models were constructed to identify factors associated with cancer progression and arthritis control. Variables included in the models were DMARD (exposure of interest), ICI and cancer type, age, sex, and time from ICI to DMARD initiation.
Results: One hundred twenty-two patients were included, mean (SD) age 62 (12) years, 39% female, 39% with melanoma, 8% non-small cell lung cancer, 19% renal cell carcinoma and 7% bladder cancer (Table 1). Seventy percent received PD(L)1 monotherapy. Median (IQR) time from ICI initiation to ICI-A onset was 170 (63,369) days, and from ICI-A onset to initiation of a DMARD 385 (258,668) days. Time from ICI initiation to DMARD start was shortest for IL6Ri-only treated patients. Median CDAI at rheumatology presentation was 22 (11,26) and 41% of the patients had other concomitant immune related adverse events. ICI were held for ICI-A in 21% of patients and discontinued in 41%. The DMARD used was a TNFi in 13%, IL6Ri 35%, MTX 31%, TNFi/MTX 9% and IL6Ri/MTX 11%. Total duration of DMARD treatment was shortest for TNFi-only treated patients. A Kaplan-Meier curve showing time to cancer progression by DMARD treatment is shown in Figure 1, and time to arthritis control in Figure 2. Compared to patients treated with a TNFi ± MTX, patients treated with IL6Ri ± MTX had a similar time to cancer progression (HR 0.74; 95%CI 0.32-1.72, P=0.49) and to arthritis control (HR 0.84; 95%CI 0.46-1.50, P=0.55). Patients treated with MTX alone had a trend toward a longer time to cancer progression (HR 0.39; 95%CI 0.13-0.1.19, P=0.10) and a longer time to arthritis control (HR 0.37; 95%CI 0.19-0.72, P=0.004) than patients treated with a TNFi ± MTX.
Conclusion: This retrospective cohort study suggests that ICI-A patients treated with either a TNFi ± MTX or an IL6Ri ± MTX have a shorter time to cancer progression but faster arthritis control than patients treated with MTX alone. A prospective randomized controlled trial is needed to verify these findings and to identify the optimal approach to managing patients with high grade ICI-A.
.jpg) Table 1. Description of the study cohort
Table 1. Description of the study cohort Figure 1. Kaplan-Meier curve showing time to cancer progression by DMARD treatment
Figure 1. Kaplan-Meier curve showing time to cancer progression by DMARD treatment Figure 2. Kaplan-Meier curve showing time to arthritis control by DMARD treatment
Figure 2. Kaplan-Meier curve showing time to arthritis control by DMARD treatmentDisclosures: A. Bass, None; N. Abdel-Wahab, None; P. Reid, provisional patent; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; C. Calabrese, Sanofi, Astrazenica; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; D. Rajesh, None; N. Ghosh, GoodRx, Musculo; K. Mushtaq, None; F. Al Haj, None; A. Falohun, None; L. Gedmintas, None; L. MacFarlane, None; S. Arabelovic, None; A. Diab, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; K. Chan, None; L. C. Cappelli, Bristol-Myers Squibb(BMS).

