Back
Plenary Session
Rheumatoid arthritis (RA)
Session: Plenary III
1680: Module Signatures of Synovial Single-cell States Identify Disease Phenotypes in Early Treatment-naive Rheumatoid Arthritis
Monday, November 14, 2022
12:45 PM – 12:55 PM Eastern Time
Location: Exhibit Hall A
- FR
Felice Rivellese, MD, PhD
Queen Mary University of London
London, United Kingdom
Presenting Author(s)
Felice Rivellese1, Elena Pontarini2, Cankut Cubuk1, Alessandra Nerviani1, Liliane Fossati-Jimack1, Anna Surace1, Elisabetta Sciacca3, Katriona Goldmann1, Michele Bombardieri4, Myles Lewis1 and Costantino Pitzalis1, 1Queen Mary University of London, London, United Kingdom, 2William Harvey Research Institute, London, United Kingdom, 3Queen Mary University of London, Biancavilla, Italy, 4Experimental Medicine and Rheumatology, William Harvey Research Institute, Queen Mary University, London, United Kingdom
Background/Purpose: Despite extensive studies, the relationship between circulating and local immune cells and clinico-pathological features of Immune-Mediated Inflammatory Diseases remains unclear. Recently, synovium-specific cell states have been described by single cell analyses, however their association with clinical outcomes remains unknown.
Methods: RNA-sequencing was performed on RNA extracted from ultrasound-guided synovial biopsies (n=49) and matched peripheral blood samples (n=36) from patients with early (< 12months) untreated RA (EULAR/ACR 2010 criteria). To assess the presence of 18 recently described synovium-specific immune cell states, we calculated module scores using their most expressed genes, and assessed their association with disease outcomes.
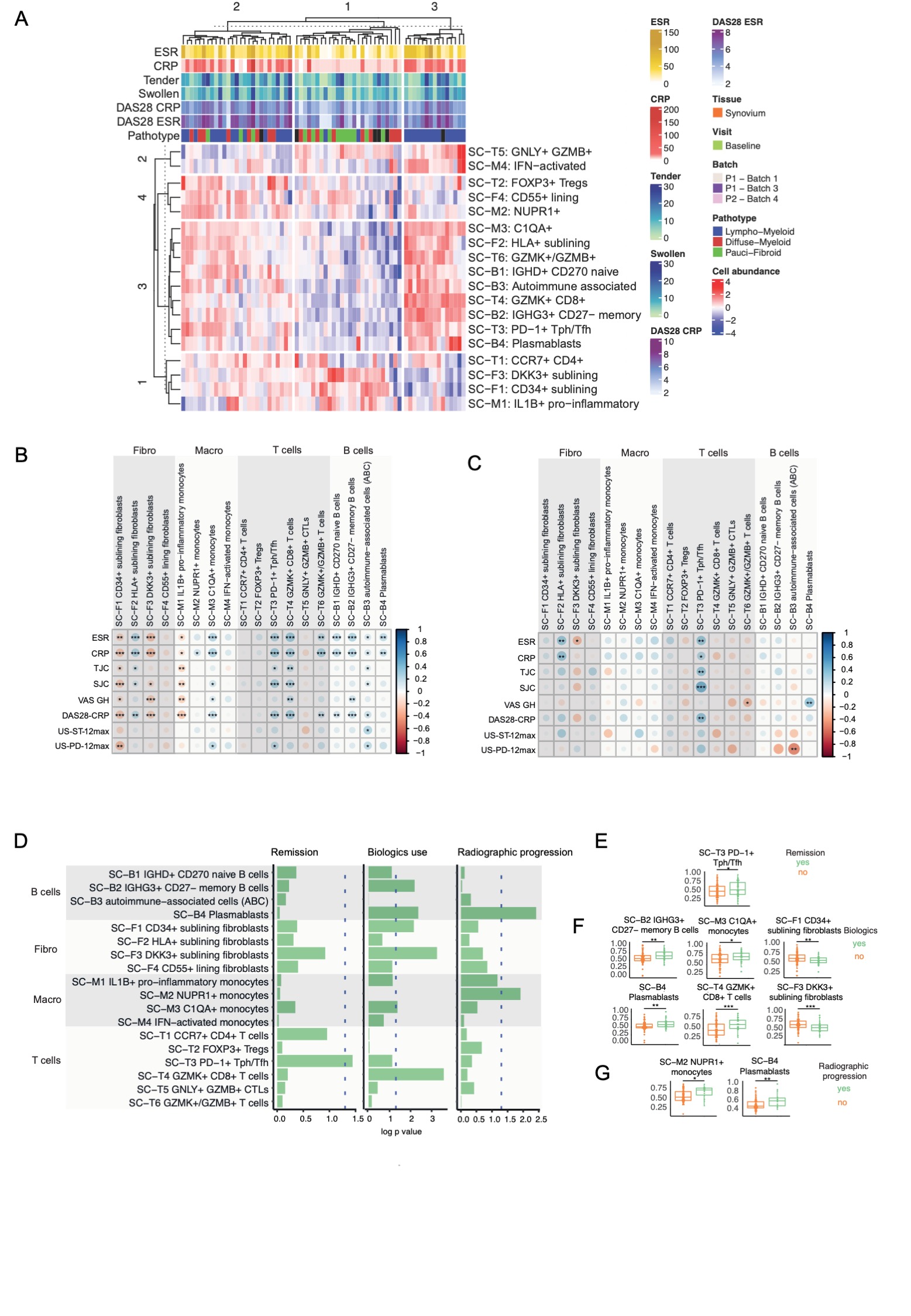
Results: Unsupervised clustering of single-cell module scores identified 3 clusters associated with synovial pathotypes (Figure 1A): cluster 3 corresponding almost exclusively to the lympho-myeloid pathotype, with enrichment of B, T cell and macrophage subsets; cluster 2, with prevalence of the fibroid/pauci-immune pathotype and enrichment of CD34+ sublining fibroblasts, DKK3+ sublining fibroblasts, and IL-1B+ pro-inflammatory macrophages; cluster 1, a mixed group, with a balanced representation of lympho-myeloid and diffuse-myeloid pathotypes/cellular subsets. Further analyses of single-cell signatures revealed a negative correlation of CD34+ sublining fibroblasts, DKK3+ sublining fibroblasts and IL-1B+ pro-inflammatory macrophages with inflammatory markers, tender and swollen joints, and disease activity scores (Figure 1B), while C1QA+ macrophages, PD1+ Tph cells, GZMK+CD8+ T cells and all B cell subsets showed strong positive correlations with inflammatory markers, disease activity and their components (Figure 1B). In the peripheral blood, significant correlations were only maintained for PD1+ Tph cells (Figure 1C). The Tph signature was also associated with clinical outcomes (Figure 1D), as patients reaching remission at 6 months in response to csDMARDs had significantly higher pre-treatment TpH levels (figure 1E). Patients who progressed to biologic DMARDs within 1 year had significantly higher baseline memory B cells (SC-B2 IGHG3+CD27-), plasmablasts, C1QA+ monocytes and GZMK+ CD8 T cells, but significantly lower CD34+ fibroblasts and DKK3+ fibroblasts (Figure 1F). Finally, radiographic progression at 1 year was associated with baseline plasmablast and NUPR1+ monocytes (Figure 1G). These findings were maintained after correcting by logistic regression for baseline demographic and clinical factors.
Conclusion: These results indicate the pre-treatment synovial single-cell modular signatures are associated with disease outcomes, including treatment response, use of biologic DMARDs and structural damage progression.
Disclosures: F. Rivellese, None; E. Pontarini, None; C. Cubuk, None; A. Nerviani, None; L. Fossati-Jimack, None; A. Surace, None; E. Sciacca, None; K. Goldmann, None; M. Bombardieri, Amgen, Janssen, GlaxoSmithKlein(GSK), UCB; M. Lewis, None; C. Pitzalis, AbbVie/Abbott, Astellas, Astra-Zeneca/MedImmune, BMS, CelGene, Grunenthal, GSK, Johnson/J&J, Kiniksa, MSD, Pfizer, Sanofi, Roche/Genentech/Chugai, UCB.
Background/Purpose: Despite extensive studies, the relationship between circulating and local immune cells and clinico-pathological features of Immune-Mediated Inflammatory Diseases remains unclear. Recently, synovium-specific cell states have been described by single cell analyses, however their association with clinical outcomes remains unknown.
Methods: RNA-sequencing was performed on RNA extracted from ultrasound-guided synovial biopsies (n=49) and matched peripheral blood samples (n=36) from patients with early (< 12months) untreated RA (EULAR/ACR 2010 criteria). To assess the presence of 18 recently described synovium-specific immune cell states, we calculated module scores using their most expressed genes, and assessed their association with disease outcomes.
Results: Unsupervised clustering of single-cell module scores identified 3 clusters associated with synovial pathotypes (Figure 1A): cluster 3 corresponding almost exclusively to the lympho-myeloid pathotype, with enrichment of B, T cell and macrophage subsets; cluster 2, with prevalence of the fibroid/pauci-immune pathotype and enrichment of CD34+ sublining fibroblasts, DKK3+ sublining fibroblasts, and IL-1B+ pro-inflammatory macrophages; cluster 1, a mixed group, with a balanced representation of lympho-myeloid and diffuse-myeloid pathotypes/cellular subsets. Further analyses of single-cell signatures revealed a negative correlation of CD34+ sublining fibroblasts, DKK3+ sublining fibroblasts and IL-1B+ pro-inflammatory macrophages with inflammatory markers, tender and swollen joints, and disease activity scores (Figure 1B), while C1QA+ macrophages, PD1+ Tph cells, GZMK+CD8+ T cells and all B cell subsets showed strong positive correlations with inflammatory markers, disease activity and their components (Figure 1B). In the peripheral blood, significant correlations were only maintained for PD1+ Tph cells (Figure 1C). The Tph signature was also associated with clinical outcomes (Figure 1D), as patients reaching remission at 6 months in response to csDMARDs had significantly higher pre-treatment TpH levels (figure 1E). Patients who progressed to biologic DMARDs within 1 year had significantly higher baseline memory B cells (SC-B2 IGHG3+CD27-), plasmablasts, C1QA+ monocytes and GZMK+ CD8 T cells, but significantly lower CD34+ fibroblasts and DKK3+ fibroblasts (Figure 1F). Finally, radiographic progression at 1 year was associated with baseline plasmablast and NUPR1+ monocytes (Figure 1G). These findings were maintained after correcting by logistic regression for baseline demographic and clinical factors.
Conclusion: These results indicate the pre-treatment synovial single-cell modular signatures are associated with disease outcomes, including treatment response, use of biologic DMARDs and structural damage progression.

Disclosures: F. Rivellese, None; E. Pontarini, None; C. Cubuk, None; A. Nerviani, None; L. Fossati-Jimack, None; A. Surace, None; E. Sciacca, None; K. Goldmann, None; M. Bombardieri, Amgen, Janssen, GlaxoSmithKlein(GSK), UCB; M. Lewis, None; C. Pitzalis, AbbVie/Abbott, Astellas, Astra-Zeneca/MedImmune, BMS, CelGene, Grunenthal, GSK, Johnson/J&J, Kiniksa, MSD, Pfizer, Sanofi, Roche/Genentech/Chugai, UCB.

