Back
Poster Session D
Session: (1980–2016) RA – Treatment Poster IV
2016: Safety and Efficacy Associated with Long-Term Low Dose Glucocorticoids in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AP
Andriko Palmowski, MD
Charité - Universitätsmedizin Berlin
Berlin, Germany
Abstract Poster Presenter(s)
Andriko Palmowski1, Sabrina Mai Nielsen2, Zhivana Boyadzhieva3, Abelina Schneider3, Anne Pankow3, Linda Hartman4, Jose Pereira da Silva5, John Kirwan6, Siegfried Wassenberg7, Christian Dejaco8, Robin Christensen9, Maarten Boers10 and Frank Buttgereit11, 1Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 2The Parker Institute, Section for Biostatistics and Evidence-Based Research, Bispebjerg-Frederiksberg Hospital, Frederiksberg, Denmark, 3Charité-Universitätsmedizin Berlin, Berlin, Germany, 4Amsterdam University Medical Centers, Vrije Universiteit, Amsterdam, Netherlands, 5University of Coimbra, Rheumatology, Columbia, Portugal, 6University of Bristol, Bristol, United Kingdom, 7Rheumazentrum Ratingen, Ratingen, Germany, 8Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Brunico, Italy, and Department of Rheumatology and Immunology, Medical University of Graz, Graz, Austria, 9Musculoskeletal Statistics Unit, The Parker Institute, Copenhagen, Denmark, 10Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 11Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin / DRFZ Berlin, Berlin, Germany
Background/Purpose: There is ongoing controversy about the safety and efficacy of long-term low dose glucocorticoids (GCs) in rheumatoid arthritis (RA). Our aim was to study the safety and efficacy associated with long-term low dose GCs in RA over two years or more.
Methods: Following a prespecified protocol (PROSPERO [CRD42021252528]), we performed a systematic review and meta-analysis of double-blind, placebo-controlled randomized trials (RCTs) comparing GCs (≤ 7.5mg/day prednisone equivalent) to placebo over a trial period of at least two years. MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception to May 1st, 2021, accompanied by a hand search. The primary outcome was the rate of adverse events (AEs). Secondary outcomes included both harm (serious AEs, death, withdrawal due to AEs, and AEs of special interest commonly associated with GC use) and a variety of benefit outcomes from the RA Core Outcome Set (e.g., disease activity score-28 joints [DAS28]), radiographic progression, and disability (measured with the health assessment questionnaire [HAQ]). Two reviewers independently screened all articles for inclusion. Unpublished data from three trials were acquired. Risk of bias was assessed with the Cochrane Risk of Bias tool. We performed random-effects meta-analyses and used GRADE to assess the quality of evidence (QoE).
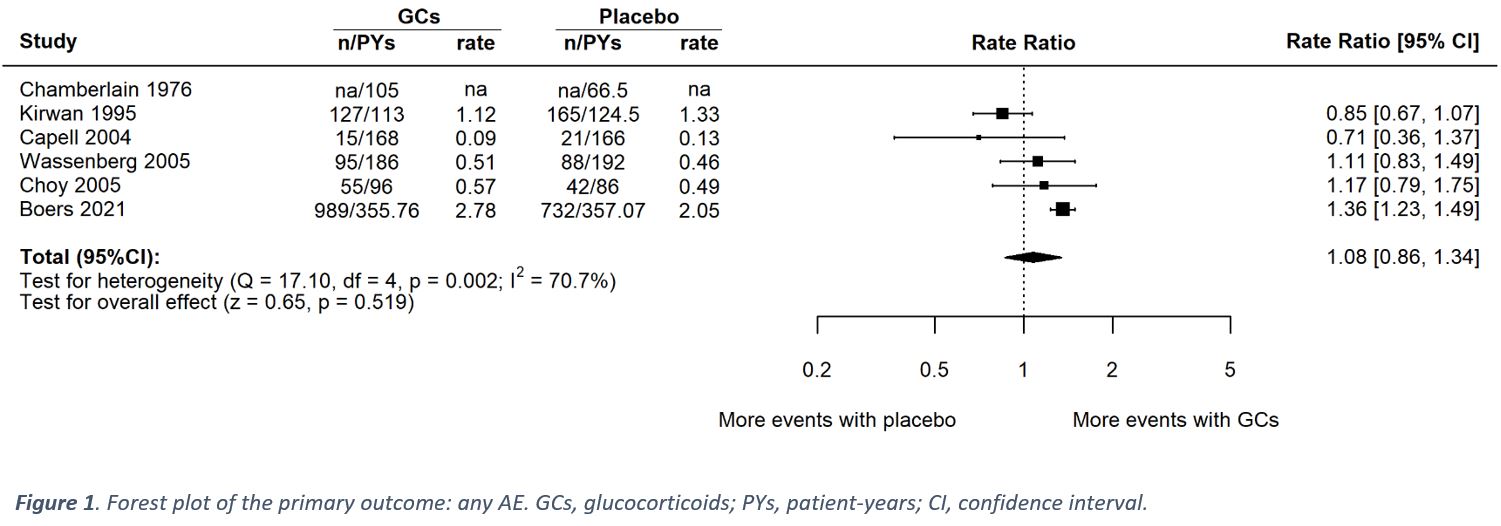
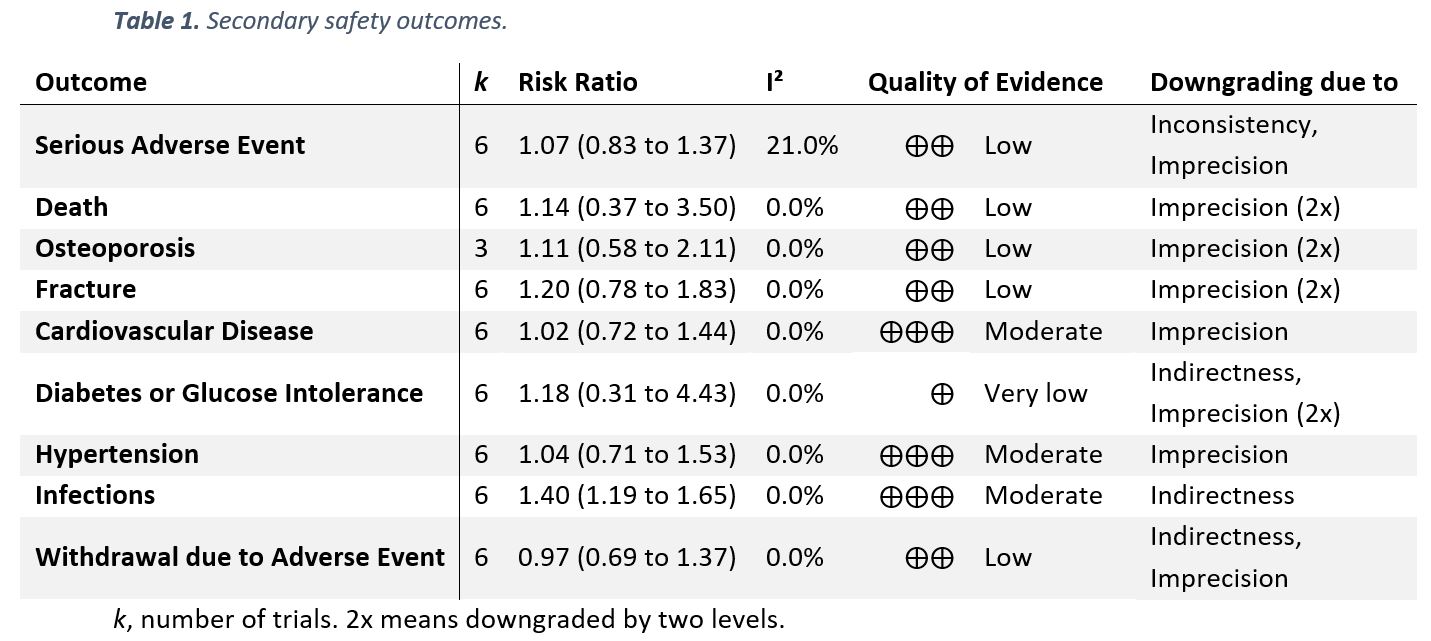
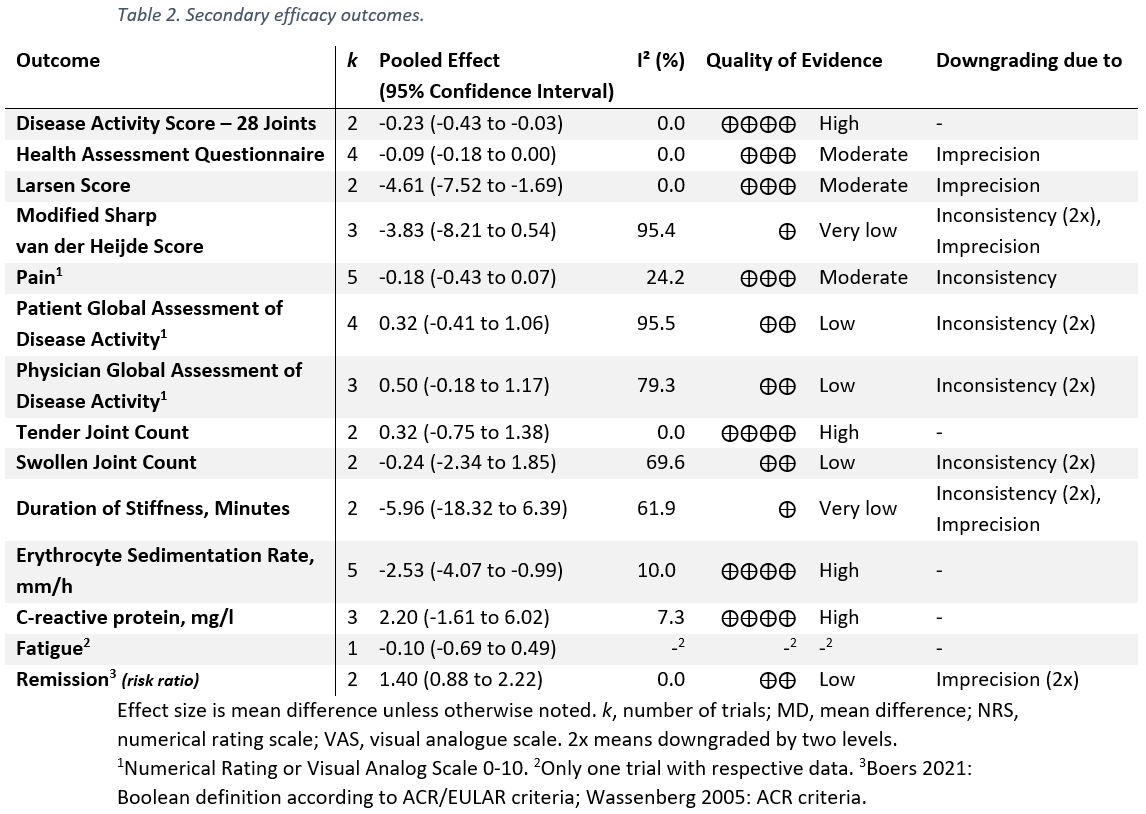
Results: Six trials with 1,078 participants were eligible for the qualitative evidence synthesis. However, only five of these RCTs presented data enabling quantitative evidence synthesis for the primary outcome. There was no evidence of an increased risk of AEs (incidence rate ratio 1.08; 95% CI 0.86 to 1.34; I² 71%; p 0.52; Figure 1), yet, QoE was low. The risks of death, serious AEs, withdrawals due to AEs, and AEs of special interest (including osteoporosis, fracture, cardiovascular disease, hypertension, and diabetes or glucose intolerance) were not different from placebo with very low to moderate QoE (Table 1); i.e., all 95% confidence Intervals indicated no potentially significant differences between groups. Infections, however, occurred more frequently in patients taking GCs (risk ratio 1.4; 1.19 to 1.65; I² 0%; moderate QoE). For benefit (Table 2), we found moderate to high quality evidence of improvement in disease activity (DAS28: -0.23, -0.43 to -0.03, I² 0%; ESR -4.07mm/h, -2.53 to -0.99, I² 10%), function (HAQ -0.09, -0.18 to 0.00, I² 0%), and radiographic progression measured by Larsen scores (-4.61, -7.52 to -1.69, I² 0%). For other efficacy outcomes, including radiographic progression measured by Sharp van der Heijde scores, there was no evidence of significant benefits with GCs.
Conclusion: There is very low to moderate quality of evidence of no harm from long-term low dose GC use in RA, except for an increased risk of infections in GC users. The benefit-risk ratio might be reasonable for using low dose long-term GCs taking into account the moderate to high quality evidence for disease-modifying properties, i.e., benefits in disease activity, function, and radiographic progression.
Disclosures: A. Palmowski, None; S. Nielsen, None; Z. Boyadzhieva, None; A. Schneider, None; A. Pankow, None; L. Hartman, None; J. Pereira da Silva, None; J. Kirwan, None; S. Wassenberg, AbbVie, Lilly, MSD, Pfizer, UCB, Galapagos, Gilead, Nichi-Iko, Sanofi; C. Dejaco, Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Galapagos and Sanofi; R. Christensen, None; M. Boers, Novartis; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer.
Background/Purpose: There is ongoing controversy about the safety and efficacy of long-term low dose glucocorticoids (GCs) in rheumatoid arthritis (RA). Our aim was to study the safety and efficacy associated with long-term low dose GCs in RA over two years or more.
Methods: Following a prespecified protocol (PROSPERO [CRD42021252528]), we performed a systematic review and meta-analysis of double-blind, placebo-controlled randomized trials (RCTs) comparing GCs (≤ 7.5mg/day prednisone equivalent) to placebo over a trial period of at least two years. MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception to May 1st, 2021, accompanied by a hand search. The primary outcome was the rate of adverse events (AEs). Secondary outcomes included both harm (serious AEs, death, withdrawal due to AEs, and AEs of special interest commonly associated with GC use) and a variety of benefit outcomes from the RA Core Outcome Set (e.g., disease activity score-28 joints [DAS28]), radiographic progression, and disability (measured with the health assessment questionnaire [HAQ]). Two reviewers independently screened all articles for inclusion. Unpublished data from three trials were acquired. Risk of bias was assessed with the Cochrane Risk of Bias tool. We performed random-effects meta-analyses and used GRADE to assess the quality of evidence (QoE).
Results: Six trials with 1,078 participants were eligible for the qualitative evidence synthesis. However, only five of these RCTs presented data enabling quantitative evidence synthesis for the primary outcome. There was no evidence of an increased risk of AEs (incidence rate ratio 1.08; 95% CI 0.86 to 1.34; I² 71%; p 0.52; Figure 1), yet, QoE was low. The risks of death, serious AEs, withdrawals due to AEs, and AEs of special interest (including osteoporosis, fracture, cardiovascular disease, hypertension, and diabetes or glucose intolerance) were not different from placebo with very low to moderate QoE (Table 1); i.e., all 95% confidence Intervals indicated no potentially significant differences between groups. Infections, however, occurred more frequently in patients taking GCs (risk ratio 1.4; 1.19 to 1.65; I² 0%; moderate QoE). For benefit (Table 2), we found moderate to high quality evidence of improvement in disease activity (DAS28: -0.23, -0.43 to -0.03, I² 0%; ESR -4.07mm/h, -2.53 to -0.99, I² 10%), function (HAQ -0.09, -0.18 to 0.00, I² 0%), and radiographic progression measured by Larsen scores (-4.61, -7.52 to -1.69, I² 0%). For other efficacy outcomes, including radiographic progression measured by Sharp van der Heijde scores, there was no evidence of significant benefits with GCs.
Conclusion: There is very low to moderate quality of evidence of no harm from long-term low dose GC use in RA, except for an increased risk of infections in GC users. The benefit-risk ratio might be reasonable for using low dose long-term GCs taking into account the moderate to high quality evidence for disease-modifying properties, i.e., benefits in disease activity, function, and radiographic progression.



Disclosures: A. Palmowski, None; S. Nielsen, None; Z. Boyadzhieva, None; A. Schneider, None; A. Pankow, None; L. Hartman, None; J. Pereira da Silva, None; J. Kirwan, None; S. Wassenberg, AbbVie, Lilly, MSD, Pfizer, UCB, Galapagos, Gilead, Nichi-Iko, Sanofi; C. Dejaco, Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Galapagos and Sanofi; R. Christensen, None; M. Boers, Novartis; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer.

