Back
Poster Session D
Immunobiology
Session: (1727–1749) T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
1745: Effects of Excess IL-18 in Mixed Inflammatory Environments
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- VD
Vinh Dang, BS
Children's Hospital of Philadelphia
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Vinh Dang1, Jemy Varghese1, Emily Landy2, Lauren Van Der Kraak2, Leonardo Huang1, Anastasia Frank-Kamenetskii1 and Scott Canna1, 1Children's Hospital of Philadelphia, Philadelphia, PA, 2University of Pittsburgh, Pittsburgh, PA
Background/Purpose: Interleukin 18 (IL-18) is an inflammasome-activated, IL-1 family cytokine that canonically induces interferongamma (IFNg). IL-18 activity is potently inhibited by a soluble, IFNg-inducible antagonist, IL-18 Binding Protein (IL-18BP). IFNg appears central to the pathogenesis of rare, hyperinflammatory states like Hemophagocytic Lymphohistiocytosis (HLH) and Macrophage Activation Syndrome (MAS), and IL-18 is both a biomarker of MAS and may be central to its pathogenesis. In some patients, MAS may compete with IFNg-independent features like arthritis, and IL-18 may contribute to both. More recently, patients with PSTPIP1 mutations causing neutrophilic skin rashes and arthritis were found to have chronic elevation of IL-18. These observations reinforce abundant evidence from model systems that IL-18 is also capable of amplifying Type 2 and 17 inflammatory responses.
Methods: C57BL/6 WT and transgenic mice underwent experimental autoimmune encephalomyelitis (EAE) and inhaled house dust mite (HDM), which are models Type 1/17 and Type 1/2 competition, respectively.
Results: Without stimulation, Il18bp-/- mice show no baseline skew towards Type1 responses except in intestinal epithelium. By contrast, Il18tg mice show a mild increase in serum IFNg and IFNg-producing cells in multiple tissues, most notably liver and spleen. Upon induction of EAE, Il18bp-/- mice were profoundly protected from weight loss, clinical score, and CNS infiltration of T-cells. This protection was lost upon systemic neutralization of IFNg, suggesting unopposed IL-18 was protective via induction of IFNg. Intestinal immune responses have been shown to affect EAE. However, mice with an Nlrc4 inflammasome mutation causing Th1-expansion and excess IL-18 restricted to the intestines were not protected from EAE. Notably, CD4 T-cell infiltration into spinal cords was not significantly different during EAE in WT and Il18bp-/- mice, but only Il18bp-/- mice showed significant infiltration of CD8 T-cells. Excess IL-18 protected against airway eosinophilia and Th2 differentiation in HDM in Il18tg but not Il18bp-/- mice, possibly due to minimal induction of IL-18 in the latter.
Conclusion: Despite IL-18's ability to amplify Type 2 and 17 responses in some circumstances, in models where T-cell differentiation states actively compete with each other, excess IL-18 prevents against non Type 1 immunopathology. This likely occurs indirectly, via the products of preferential-enhancement of type 1 responses. This may be relevant to therapeutics aimed at blocking pathogenic Th2 or 17 responses or in predicting the consequences of IL-18 blockade in complex inflammatory states.
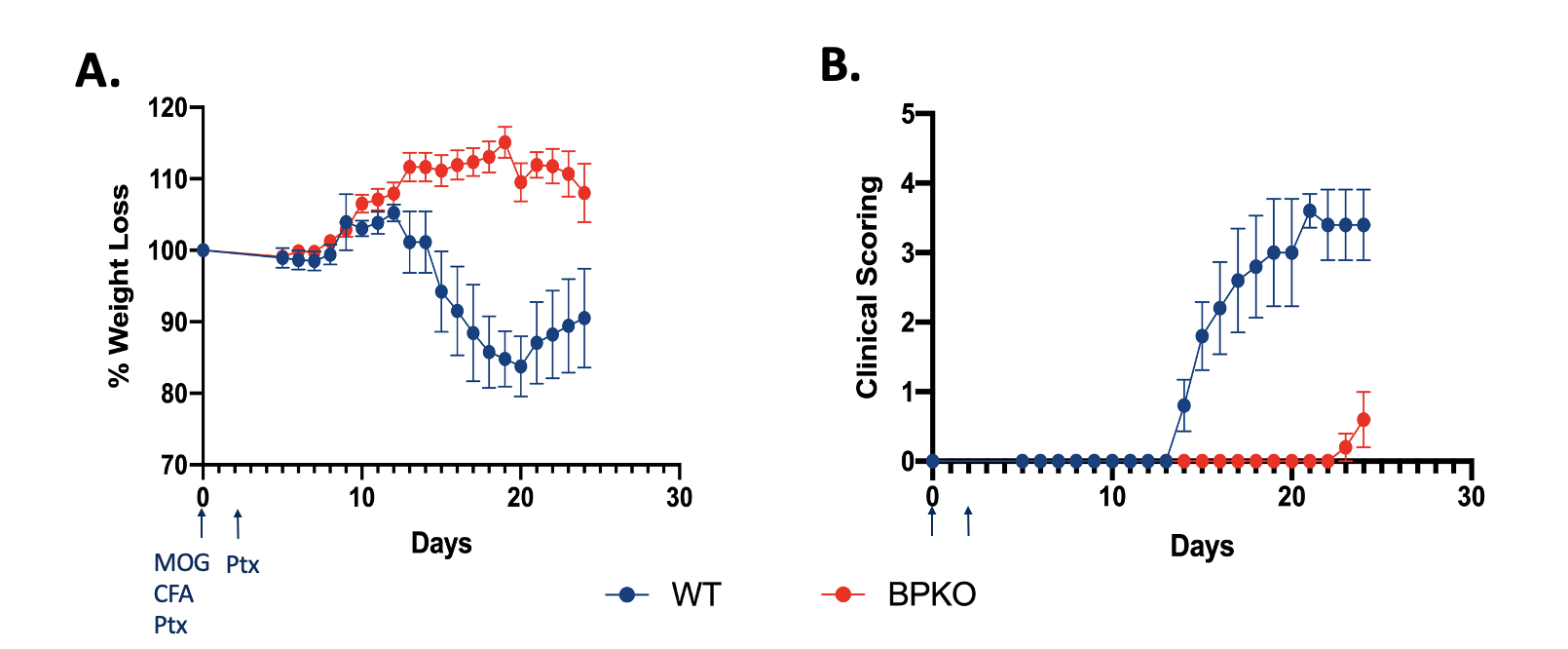 Unopposed IL-18 protects mice from experiencing clinical outcomes of EAE 24 days post immunization with MOG peptide and Pertussis Toxin. (A) Percent weight loss of Il18bp-/- mice in comparison to WT mice. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice. (n=5)
Unopposed IL-18 protects mice from experiencing clinical outcomes of EAE 24 days post immunization with MOG peptide and Pertussis Toxin. (A) Percent weight loss of Il18bp-/- mice in comparison to WT mice. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice. (n=5)
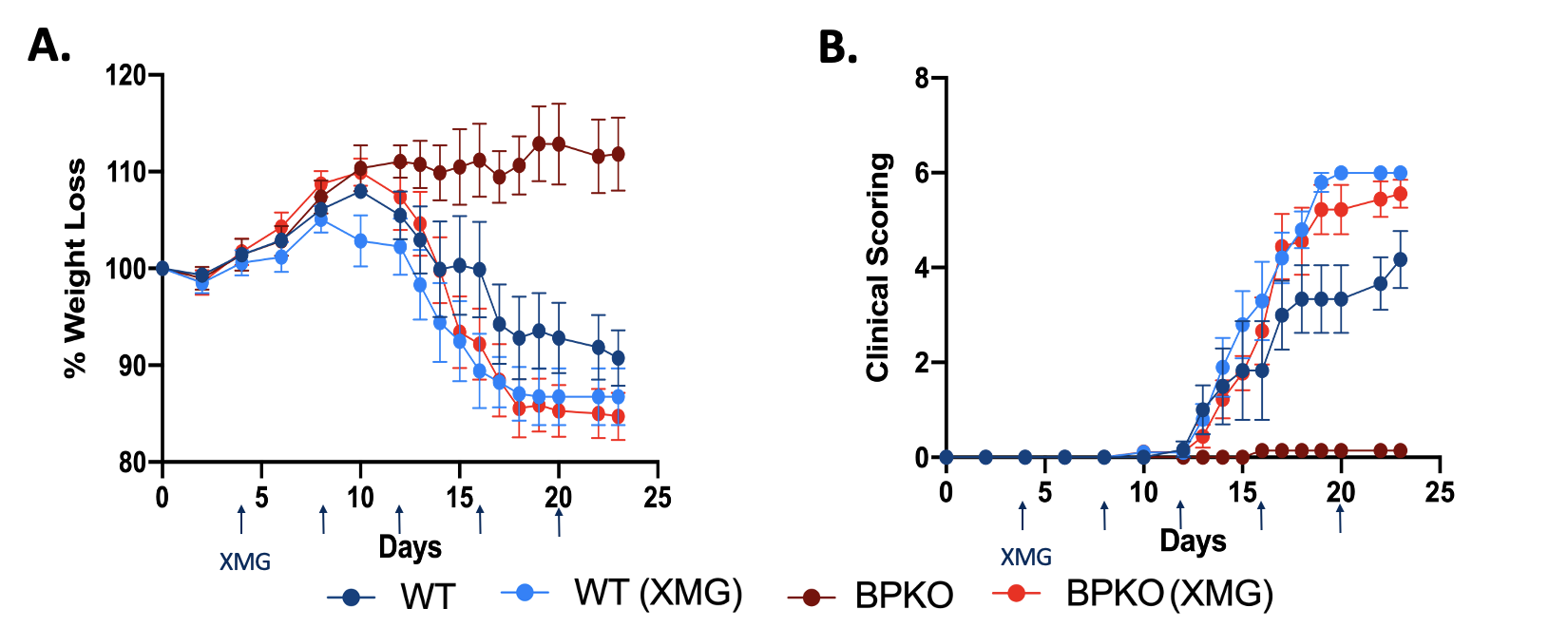 Blocking IFNg in Il18bp-/- mice renders mice susceptible to EAE meaning the protection offered by unopposed IL-18 is IFNg dependent. (A) Percent weight loss of Il-18bpKO mice in comparison to WT mice untreated and treated with XMG 1.2. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice untreated and treated with XMG 1.2. (n=5)
Blocking IFNg in Il18bp-/- mice renders mice susceptible to EAE meaning the protection offered by unopposed IL-18 is IFNg dependent. (A) Percent weight loss of Il-18bpKO mice in comparison to WT mice untreated and treated with XMG 1.2. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice untreated and treated with XMG 1.2. (n=5)
Disclosures: V. Dang, None; J. Varghese, None; E. Landy, None; L. Van Der Kraak, None; L. Huang, None; A. Frank-Kamenetskii, None; S. Canna, Sobi, Ab2bio, Novartis, Immvention Therapeutics, sobi, Simcha Therapeutics.
Background/Purpose: Interleukin 18 (IL-18) is an inflammasome-activated, IL-1 family cytokine that canonically induces interferongamma (IFNg). IL-18 activity is potently inhibited by a soluble, IFNg-inducible antagonist, IL-18 Binding Protein (IL-18BP). IFNg appears central to the pathogenesis of rare, hyperinflammatory states like Hemophagocytic Lymphohistiocytosis (HLH) and Macrophage Activation Syndrome (MAS), and IL-18 is both a biomarker of MAS and may be central to its pathogenesis. In some patients, MAS may compete with IFNg-independent features like arthritis, and IL-18 may contribute to both. More recently, patients with PSTPIP1 mutations causing neutrophilic skin rashes and arthritis were found to have chronic elevation of IL-18. These observations reinforce abundant evidence from model systems that IL-18 is also capable of amplifying Type 2 and 17 inflammatory responses.
Methods: C57BL/6 WT and transgenic mice underwent experimental autoimmune encephalomyelitis (EAE) and inhaled house dust mite (HDM), which are models Type 1/17 and Type 1/2 competition, respectively.
Results: Without stimulation, Il18bp-/- mice show no baseline skew towards Type1 responses except in intestinal epithelium. By contrast, Il18tg mice show a mild increase in serum IFNg and IFNg-producing cells in multiple tissues, most notably liver and spleen. Upon induction of EAE, Il18bp-/- mice were profoundly protected from weight loss, clinical score, and CNS infiltration of T-cells. This protection was lost upon systemic neutralization of IFNg, suggesting unopposed IL-18 was protective via induction of IFNg. Intestinal immune responses have been shown to affect EAE. However, mice with an Nlrc4 inflammasome mutation causing Th1-expansion and excess IL-18 restricted to the intestines were not protected from EAE. Notably, CD4 T-cell infiltration into spinal cords was not significantly different during EAE in WT and Il18bp-/- mice, but only Il18bp-/- mice showed significant infiltration of CD8 T-cells. Excess IL-18 protected against airway eosinophilia and Th2 differentiation in HDM in Il18tg but not Il18bp-/- mice, possibly due to minimal induction of IL-18 in the latter.
Conclusion: Despite IL-18's ability to amplify Type 2 and 17 responses in some circumstances, in models where T-cell differentiation states actively compete with each other, excess IL-18 prevents against non Type 1 immunopathology. This likely occurs indirectly, via the products of preferential-enhancement of type 1 responses. This may be relevant to therapeutics aimed at blocking pathogenic Th2 or 17 responses or in predicting the consequences of IL-18 blockade in complex inflammatory states.
 Unopposed IL-18 protects mice from experiencing clinical outcomes of EAE 24 days post immunization with MOG peptide and Pertussis Toxin. (A) Percent weight loss of Il18bp-/- mice in comparison to WT mice. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice. (n=5)
Unopposed IL-18 protects mice from experiencing clinical outcomes of EAE 24 days post immunization with MOG peptide and Pertussis Toxin. (A) Percent weight loss of Il18bp-/- mice in comparison to WT mice. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice. (n=5) Blocking IFNg in Il18bp-/- mice renders mice susceptible to EAE meaning the protection offered by unopposed IL-18 is IFNg dependent. (A) Percent weight loss of Il-18bpKO mice in comparison to WT mice untreated and treated with XMG 1.2. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice untreated and treated with XMG 1.2. (n=5)
Blocking IFNg in Il18bp-/- mice renders mice susceptible to EAE meaning the protection offered by unopposed IL-18 is IFNg dependent. (A) Percent weight loss of Il-18bpKO mice in comparison to WT mice untreated and treated with XMG 1.2. (n=5) (B) Clinical outcomes of Il18bp-/- mice compared to WT mice untreated and treated with XMG 1.2. (n=5)Disclosures: V. Dang, None; J. Varghese, None; E. Landy, None; L. Van Der Kraak, None; L. Huang, None; A. Frank-Kamenetskii, None; S. Canna, Sobi, Ab2bio, Novartis, Immvention Therapeutics, sobi, Simcha Therapeutics.

