Back
Poster Session D
Session: (1980–2016) RA – Treatment Poster IV
2011: More Meticulously Following Treat-to-target in RA Does Not Lead to Less Radiographic Progression: A Longitudinal Analysis in BIODAM
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Sofia Ramiro, MD, PhD
Leiden University Medical Center
Bunde, Netherlands
Abstract Poster Presenter(s)
Sofia Ramiro1, Robert Landewé2, Désirée van der Heijde3, Alexandre Sepriano4, Oliver FitzGerald5, Mikkel Østergaard6, Joanne Homik7, Ori Elkayam8, Carter Thorne9, Maggie Larche10, Gianfranco Ferraccioli11, Marina Backhaus12, Gilles Boire13, Bernard Combe14, Thierry Schaeverbeke15, Alain Saraux16, Maxime Dougados17, Maurizio Rossini18, Marcello Govoni19, Luigi Sinigaglia20, Alain Cantagrel21, CF Allaart1, Cheryl Barnabe22, Clifton O. Bingham III23, Dirkjan van Schaardenburg24, HIlde Hammer25, Rana Dadashova26, Edna Hutchings26, Joel Paschke26 and Walter P Maksymowych27, 1Leiden University Medical Center, Leiden, Netherlands, 2Amsterdam University Medical Center, Meerssen, Netherlands, 3Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Netherlands, 4Leiden University Medical Centre, Portela Loures, Portugal, 5Conway Institute, University College Dublin, Dublin, Ireland, 6Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 7Division of Rheumatology, University of Alberta, Edmonton, AB, Canada, 8Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 9Southlake Regional Health Centre, Newmarket, ON, Canada, 10McMaster University, Hamilton, ON, Canada, 11Catholic University of the Sacred Heart, Rome, Roma, Italy, 12Park-Klinik Weissensee Academic Hospital of the Charité, Berlin, Germany, 13Universite de Sherbrooke, Sherbrooke, QC, Canada, 14CHU Montpellier and Montpellier University, Monpellier, France, 15FHU ACRONIM, University Hospital of Bordeaux, University of Bordeaux, Bordeaux, France, 16CHU Brest, Brest, France, 17Department of Rheumatology, Hôpital Cochin, Paris, France, Paris, France, 18Rheumatology Unit, Department of Medicine, University of Verona, Verona, Italy, 19S. Anna Hospital and University of Ferrara, Ferrara, Italy, 20Gaetano Pini Institute, Milano, Italy, 21CHU Toulouse, Paul Sabatier University, Toulouse, France, 22University of Calgary, Calgary, AB, Canada, 23Johns Hopkins University, Baltimore, MD, 24Amsterdam UMC, Amsterdam, Netherlands, 25Diakonhjemmet Hospital, Oslo, Oslo, Norway, 26CARE Arthritis LTD, Edmonton, AB, Canada, 27Department of Medicine, University of Alberta, Edmonton, AB, Canada
Background/Purpose: A Treat-to-Target approach (T2T) is broadly considered to lead to better clinical outcomes and recommended in patients with RA. However, very few studies have analyzed the effect of T2T on radiographic progression, and any such studies have provided inconsistent results. Our aim was to investigate whether meticulously following a treat-to-target (T2T)-strategy in daily clinical practice leads to lower radiographic progression in RA.
Methods: Patients from the multicenter RA-BIODAM cohort with ≥2 consecutive visits with radiographs available were included. In RA-BIODAM patients were enrolled as they were initiating a new csDMARD/bDMARD treatment were followed-up with the intention to benchmark and intensify treatment. The primary outcome of this analysis was the change in Sharp-van der Heijde score (SvdH, 0-448), assessed every 6 months, using average scores from 2 readers (scores with known chronological order). Following a DAS44-T2T remission strategy, which was defined at each 3-month visit, was the main variable of interest. Patients were categorized based on the proportion of visits in which T2T was followed according to our definition: very low (≤40% of the visits, low ( >40%, < 62.5%), high (≥62.5%, ≤75%) and very high ( >75%). Radiographic progression at 2 years was visualized across groups by cumulative probability plots. Per 3-month interval T2T could be followed zero, one or two times (in a total of 2 visits). Associations between the number of visits with T2T in an interval and radiographic progression, both in the same and in the subsequent 6-month interval, were analysed by generalised estimating equations, adjusted for age, gender, disease duration and country.
Results: In total, 511 patients were included (mean (SD) age: 56 (13) years; 76% female). After 2 years, patients showed on average 2.2 (4.1) units progression (median:1 unit). Mean (SD) 2-year progression was not significantly different across categories of T2T: very low: 2.1 (2.7)-units; low: 2.8 (6.0); high: 2.4 (4.5), very high: 1.6 (2.2) (Figure). Meticulously following-up T2T in a 3-month interval neither reduced progression in the same 6-month interval (parameter estimates (for yes vs no): +0.15 units (95%CI: -0.04 to 0.33) for 2 vs 0 visits; and +0.08 units (-0.06;0.22) for 1 vs 0 visits) nor did it reduce progression in the subsequent 6-month interval (Table).
Conclusion: In this daily practice cohort, more meticulously following T2T principles did not result in more reduction of radiographic progression than a somewhat more liberal attitude toward T2T. One possible interpretation of these results is that the intention to apply T2T already suffices and that a more stringent approach does not further improve outcome.
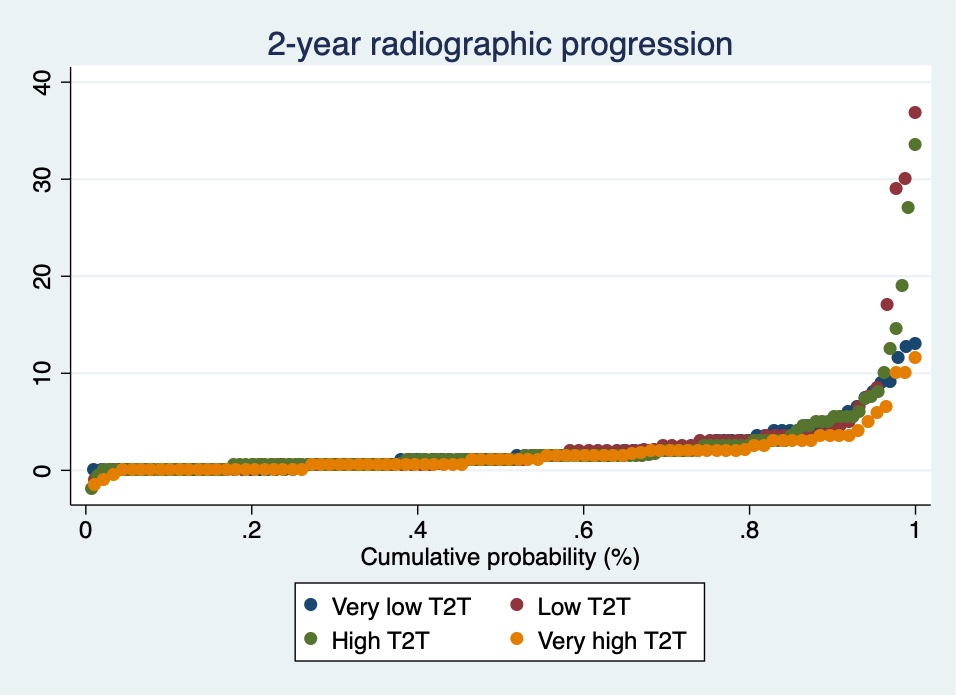 Figure 1: Cumulative probability plot with 2-year radiographic progression according to the proportion of 3-monthly visits with T2T followed
Figure 1: Cumulative probability plot with 2-year radiographic progression according to the proportion of 3-monthly visits with T2T followed
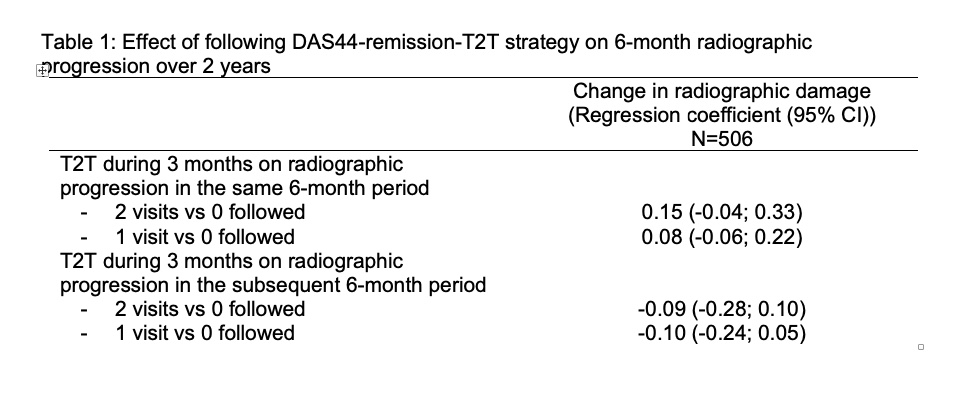 Table 1: Effect of following DAS44-remission-T2T strategy on 6-month radiographic progression over 2 years
Table 1: Effect of following DAS44-remission-T2T strategy on 6-month radiographic progression over 2 years
Disclosures: S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; A. Sepriano, UCB, Novartis; O. FitzGerald, Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer Inc, Eli Lilly, AbbVie, Janssen; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; J. Homik, AbbVie/Abbott, Amgen, Pfizer, Janssen, Merck/MSD; O. Elkayam, Pfizer, Eli Lilly, AbbVie/Abbott, Novartis, Janssen, Boehringer-Ingelheim; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; M. Larche, None; G. Ferraccioli, None; M. Backhaus, None; G. Boire, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Orimed Pharma, Pfizer, Samsung Bioepis, Teva, Viatris, Amgen, Celgene; B. Combe, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Celltrion, Galapagos, Gilead, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche; T. Schaeverbeke, None; A. Saraux, None; M. Dougados, Novartis, AbbVie, Eli Lilly, Merck, Pfizer, UCB Pharma; M. Rossini, Theramex, UCB, Amgen, AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Novartis, Pfizer Inc, Sandoz; M. Govoni, None; L. Sinigaglia, None; A. Cantagrel, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Médac, Merck/MSD, Novartis, Pfizer; C. Allaart, None; C. Barnabe, Janssen, Fresenius Kabi, Celltrion Healthcare, Pfizer; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; D. van Schaardenburg, None; H. Hammer, None; R. Dadashova, None; E. Hutchings, None; J. Paschke, None; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited.
Background/Purpose: A Treat-to-Target approach (T2T) is broadly considered to lead to better clinical outcomes and recommended in patients with RA. However, very few studies have analyzed the effect of T2T on radiographic progression, and any such studies have provided inconsistent results. Our aim was to investigate whether meticulously following a treat-to-target (T2T)-strategy in daily clinical practice leads to lower radiographic progression in RA.
Methods: Patients from the multicenter RA-BIODAM cohort with ≥2 consecutive visits with radiographs available were included. In RA-BIODAM patients were enrolled as they were initiating a new csDMARD/bDMARD treatment were followed-up with the intention to benchmark and intensify treatment. The primary outcome of this analysis was the change in Sharp-van der Heijde score (SvdH, 0-448), assessed every 6 months, using average scores from 2 readers (scores with known chronological order). Following a DAS44-T2T remission strategy, which was defined at each 3-month visit, was the main variable of interest. Patients were categorized based on the proportion of visits in which T2T was followed according to our definition: very low (≤40% of the visits, low ( >40%, < 62.5%), high (≥62.5%, ≤75%) and very high ( >75%). Radiographic progression at 2 years was visualized across groups by cumulative probability plots. Per 3-month interval T2T could be followed zero, one or two times (in a total of 2 visits). Associations between the number of visits with T2T in an interval and radiographic progression, both in the same and in the subsequent 6-month interval, were analysed by generalised estimating equations, adjusted for age, gender, disease duration and country.
Results: In total, 511 patients were included (mean (SD) age: 56 (13) years; 76% female). After 2 years, patients showed on average 2.2 (4.1) units progression (median:1 unit). Mean (SD) 2-year progression was not significantly different across categories of T2T: very low: 2.1 (2.7)-units; low: 2.8 (6.0); high: 2.4 (4.5), very high: 1.6 (2.2) (Figure). Meticulously following-up T2T in a 3-month interval neither reduced progression in the same 6-month interval (parameter estimates (for yes vs no): +0.15 units (95%CI: -0.04 to 0.33) for 2 vs 0 visits; and +0.08 units (-0.06;0.22) for 1 vs 0 visits) nor did it reduce progression in the subsequent 6-month interval (Table).
Conclusion: In this daily practice cohort, more meticulously following T2T principles did not result in more reduction of radiographic progression than a somewhat more liberal attitude toward T2T. One possible interpretation of these results is that the intention to apply T2T already suffices and that a more stringent approach does not further improve outcome.
 Figure 1: Cumulative probability plot with 2-year radiographic progression according to the proportion of 3-monthly visits with T2T followed
Figure 1: Cumulative probability plot with 2-year radiographic progression according to the proportion of 3-monthly visits with T2T followed Table 1: Effect of following DAS44-remission-T2T strategy on 6-month radiographic progression over 2 years
Table 1: Effect of following DAS44-remission-T2T strategy on 6-month radiographic progression over 2 yearsDisclosures: S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; A. Sepriano, UCB, Novartis; O. FitzGerald, Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer Inc, Eli Lilly, AbbVie, Janssen; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; J. Homik, AbbVie/Abbott, Amgen, Pfizer, Janssen, Merck/MSD; O. Elkayam, Pfizer, Eli Lilly, AbbVie/Abbott, Novartis, Janssen, Boehringer-Ingelheim; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; M. Larche, None; G. Ferraccioli, None; M. Backhaus, None; G. Boire, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Orimed Pharma, Pfizer, Samsung Bioepis, Teva, Viatris, Amgen, Celgene; B. Combe, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Celltrion, Galapagos, Gilead, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche; T. Schaeverbeke, None; A. Saraux, None; M. Dougados, Novartis, AbbVie, Eli Lilly, Merck, Pfizer, UCB Pharma; M. Rossini, Theramex, UCB, Amgen, AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Novartis, Pfizer Inc, Sandoz; M. Govoni, None; L. Sinigaglia, None; A. Cantagrel, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Médac, Merck/MSD, Novartis, Pfizer; C. Allaart, None; C. Barnabe, Janssen, Fresenius Kabi, Celltrion Healthcare, Pfizer; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; D. van Schaardenburg, None; H. Hammer, None; R. Dadashova, None; E. Hutchings, None; J. Paschke, None; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited.

