Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1798: Health-Related Quality of Life Improvements Resulting from a Treat-to-Target Strategy in the Management of Gout: Post- Hoc Analysis of a Multicenter, Randomized, Double-Blind, Non-Inferiority Trial
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LH
Lindsay Helget, MD
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Lindsay Helget1, James O'Dell1, Jeff Newcomb1, Maria Androsenko2, Mary Brophy2, Anne Davis-Karim3, Bryant England1, Ryan Ferguson2, Michael Pillinger4, Tuhina Neogi5, Paul Palevsky6, Hongsheng Wu2 and Ted Mikuls7, 1University of Nebraska Medical Center, Omaha, NE, 2VA Boston Cooperative Studies Program Coordinating Center, Boston, MA, 3VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM, 4NYU Grossman School of Medicine, New York, NY, 5Boston University School of Medicine, Boston, MA, 6University of Pittsburgh School of Medicine, Pittsburgh, Pittsburgh, 7Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: The ACR recommends a treat-to-target strategy in gout management, centered on the titration of urate lowering therapy (ULT) to a goal serum urate (SU) of < 6.0mg/dL. However, there is a paucity of data regarding improvements in health-related quality of life (HRQoL) when receiving a treat-to-target regimen for gout management. Using data from the recently completed multicenter, randomized, double-blind, STOP Gout Study (O'Dell JR et al. NEJM Evidence 2022), we examined changes in HRQoL accompanying ULT administered as part of a treat-to-target strategy.
Methods: Participants with gout and SU concentration ≥6.8 mg/dL were randomized 1:1 to receive allopurinol or febuxostat. ULT was titrated during weeks 0-24 (Phase 1) and maintained during weeks 25-48 (Phase 2), with escalation as necessary, to reach goal SU of < 6.0 mg/dL. Participants were observed on a stable ULT dose during weeks 49-72 (Phase 3). For this analysis, participants were considered non-responders to ULT (either allopurinol or febuxostat based on randomization) if they did not achieve a goal SU of < 6.0mg/dL in Phase 2 (mean levels at weeks 36, 42 and 48) despite maximal ULT dosing. The EQ-5D-3L and VR-12 (a Veteran's Administration HRQoL outcome based of the SF-12) questionnaires were collected and compared at baseline, 24 weeks, and 48 weeks and differences were examined over these time points using a repeated measures ANOVA. Baseline HRQoL values were compared by responder status using a t-test. Associations of SU change between baseline and 48 weeks, and SU goal achievement at 48 weeks with change in HRQoL outcomes were examined using linear regression models.
Results: Of the 940 trial participants, 764 had SU response data available at week 48 and were included in this analysis; 618 (80.9%) of these achieved a target SU goal < 6.0 mg/dl. Participants deemed non-responders at 48 weeks reported lower HRQoL scores at baseline when compared to responders (p< 0.001). Improvements in both measures were similar between allopurinol and febuxostat treatment arms (p >0.7 for both HRQoL measures). From baseline to 48 weeks both EQ-5D-3L and VR-12 questionnaire scores improved significantly in all participants (p< 0.0001) (Table 1). Neither SU change nor responder status (achievement of SU goal) at 48 weeks were associated with changes in HRQoL (Table 2).
Conclusion: In this secondary analysis from a large, randomized double-blind, non-inferiority trial comparing the efficacy and safety of allopurinol and febuxostat in the management of gout, we have found that HRQoL outcome measures improved with treat-to-target ULT. Over this period of observation, SU change achieved was not associated with HRQoL improvement, suggesting that a longer follow up period may be needed to identify these associations and/or that HRQoL improvements may be more closely tied with other gout outcomes such as recurrent flare. Regardless, these findings demonstrate that patients receiving treat-to-target ULT experience significant HRQoL gains even early in the course of management, supporting current ACR practice guidelines.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290920-1-ANY.jpg width=440 height=247.5 border=0 style=border-style: none;>
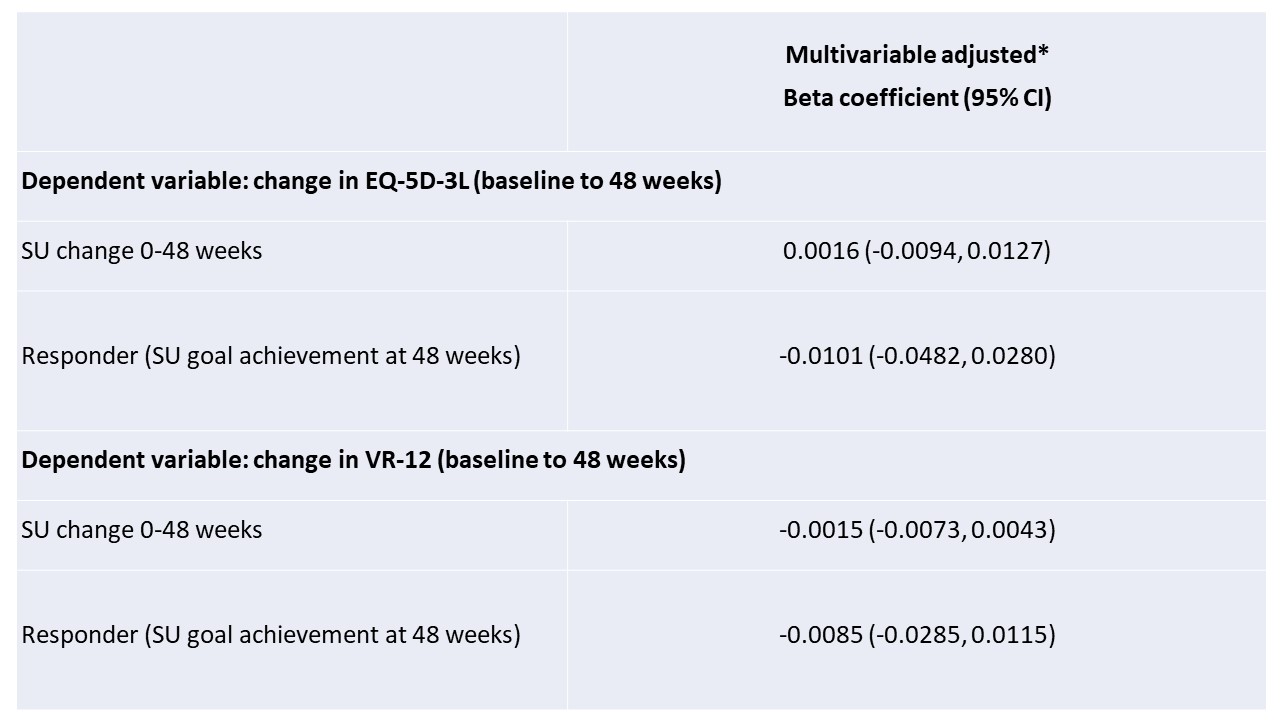 Table 2. Associations of SU change from baseline to 48 weeks and Responder Status (SU goal achievement) with change in EQ-5D-3L and VR-12; estimates generated using linear regression analysis
Table 2. Associations of SU change from baseline to 48 weeks and Responder Status (SU goal achievement) with change in EQ-5D-3L and VR-12; estimates generated using linear regression analysis
*Covariates include age, race/ethnicity (white, African American, other), chronic kidney disease, hypertension, diabetes, cardiovascular disease, body mass index, baseline SU, and treatment assignment.
Disclosures: L. Helget, None; J. O'Dell, None; J. Newcomb, None; M. Androsenko, None; M. Brophy, None; A. Davis-Karim, None; B. England, Boehringer-Ingelheim; R. Ferguson, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; P. Palevsky, None; H. Wu, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.
Background/Purpose: The ACR recommends a treat-to-target strategy in gout management, centered on the titration of urate lowering therapy (ULT) to a goal serum urate (SU) of < 6.0mg/dL. However, there is a paucity of data regarding improvements in health-related quality of life (HRQoL) when receiving a treat-to-target regimen for gout management. Using data from the recently completed multicenter, randomized, double-blind, STOP Gout Study (O'Dell JR et al. NEJM Evidence 2022), we examined changes in HRQoL accompanying ULT administered as part of a treat-to-target strategy.
Methods: Participants with gout and SU concentration ≥6.8 mg/dL were randomized 1:1 to receive allopurinol or febuxostat. ULT was titrated during weeks 0-24 (Phase 1) and maintained during weeks 25-48 (Phase 2), with escalation as necessary, to reach goal SU of < 6.0 mg/dL. Participants were observed on a stable ULT dose during weeks 49-72 (Phase 3). For this analysis, participants were considered non-responders to ULT (either allopurinol or febuxostat based on randomization) if they did not achieve a goal SU of < 6.0mg/dL in Phase 2 (mean levels at weeks 36, 42 and 48) despite maximal ULT dosing. The EQ-5D-3L and VR-12 (a Veteran's Administration HRQoL outcome based of the SF-12) questionnaires were collected and compared at baseline, 24 weeks, and 48 weeks and differences were examined over these time points using a repeated measures ANOVA. Baseline HRQoL values were compared by responder status using a t-test. Associations of SU change between baseline and 48 weeks, and SU goal achievement at 48 weeks with change in HRQoL outcomes were examined using linear regression models.
Results: Of the 940 trial participants, 764 had SU response data available at week 48 and were included in this analysis; 618 (80.9%) of these achieved a target SU goal < 6.0 mg/dl. Participants deemed non-responders at 48 weeks reported lower HRQoL scores at baseline when compared to responders (p< 0.001). Improvements in both measures were similar between allopurinol and febuxostat treatment arms (p >0.7 for both HRQoL measures). From baseline to 48 weeks both EQ-5D-3L and VR-12 questionnaire scores improved significantly in all participants (p< 0.0001) (Table 1). Neither SU change nor responder status (achievement of SU goal) at 48 weeks were associated with changes in HRQoL (Table 2).
Conclusion: In this secondary analysis from a large, randomized double-blind, non-inferiority trial comparing the efficacy and safety of allopurinol and febuxostat in the management of gout, we have found that HRQoL outcome measures improved with treat-to-target ULT. Over this period of observation, SU change achieved was not associated with HRQoL improvement, suggesting that a longer follow up period may be needed to identify these associations and/or that HRQoL improvements may be more closely tied with other gout outcomes such as recurrent flare. Regardless, these findings demonstrate that patients receiving treat-to-target ULT experience significant HRQoL gains even early in the course of management, supporting current ACR practice guidelines.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290920-1-ANY.jpg width=440 height=247.5 border=0 style=border-style: none;>
Table 1. EQ-5D-3L and VR-12 values among trial participants
*Values are presented as EQ-5D-3L or VR-12 scores means (SD); p-values generated using repeated measures ANOVA
*Treatment status not associated with HRQoL changes over 48 weeks (p>0.7)
*Values are presented as EQ-5D-3L or VR-12 scores means (SD); p-values generated using repeated measures ANOVA
*Treatment status not associated with HRQoL changes over 48 weeks (p>0.7)
 Table 2. Associations of SU change from baseline to 48 weeks and Responder Status (SU goal achievement) with change in EQ-5D-3L and VR-12; estimates generated using linear regression analysis
Table 2. Associations of SU change from baseline to 48 weeks and Responder Status (SU goal achievement) with change in EQ-5D-3L and VR-12; estimates generated using linear regression analysis*Covariates include age, race/ethnicity (white, African American, other), chronic kidney disease, hypertension, diabetes, cardiovascular disease, body mass index, baseline SU, and treatment assignment.
Disclosures: L. Helget, None; J. O'Dell, None; J. Newcomb, None; M. Androsenko, None; M. Brophy, None; A. Davis-Karim, None; B. England, Boehringer-Ingelheim; R. Ferguson, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; T. Neogi, Novartis, Pfizer/Lilly, Regeneron; P. Palevsky, None; H. Wu, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.

