Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1780: Improved Overall Survival in Patients with Lung Cancer and Autoimmune Rheumatic Diseases
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- PG
Paola Ghanem, MD
Johns Hopkins University
Baltimore, MD, United States
Abstract Poster Presenter(s)
Paola Ghanem1, Joseph C. Murray1, Christopher Mecoli2, Ami Shah3, Benjamin Levy1, Kristen A. Marrone1, David Ettinger1, Valsamo Anagnostou1, Vincent K. Lam1, Julie R. Brahmer1 and Laura C. Cappelli4, 1Johns Hopkins University, Baltimore, MD, 2Johns Hopkins University School of Medicine, Baltimore, MD, 3Johns Hopkins Rheumatology, Baltimore, MD, 4Johns Hopkins School of Medicine, Baltimore, MD
Background/Purpose: Lung cancer remains the leading cause of cancer-related death. Concomitant autoimmune diseases (AD) can add morbidity and complicate treatment decisions. We evaluated the tumor characteristics at diagnosis and clinical outcomes in lung cancer patients with and without rheumatic AD.
Methods: This is a retrospective cohort study of 10,963 patients with lung cancer from 2004 till 2021, treated at Johns Hopkins Hospital (IRB00276019). Clinical data was extracted from the institutional cancer registry and included demographic features, tumor characteristics, and clinical outcomes. Data on patients' history of 20 autoimmune rheumatic diseases was extracted from the electronic medical record based on ICD10 codes. Chi-square tests and independent t-tests were used to evaluate the association between patient and tumor characteristics and history of AD. To further evaluate the associations between tumor characteristics at diagnosis and AD, we performed multivariate logistic regression accounting for potentially confounding patient characteristics. Kaplan-Meier curves and Cox proportional hazards models were used to compare the overall survival of lung cancer patients with and without AD, adjusting for treatment modalities, age, stage and the treatment modalities (surgery, chemotherapy, immunotherapy, radiation therapy).
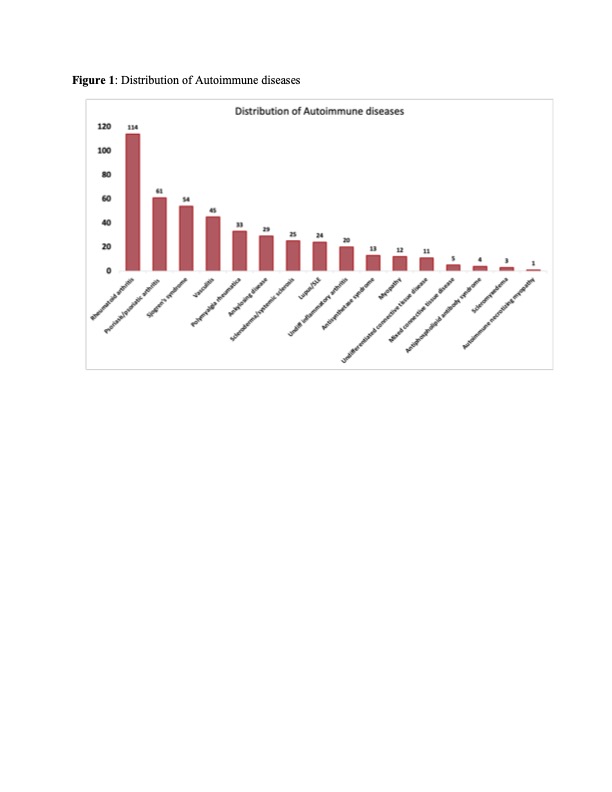
Results: AD was documented in 3.6% of lung cancer patients (n=454); the most common AD was rheumatoid arthritis followed by psoriasis/psoriatic arthritis (Figure 1). The age at the time of lung cancer diagnosis was 69 ± 10 and 67 ± 12 years in patients with and without AD, respectively (p= 0.02). Female sex and former smoking status were found to be significantly associated with a history of AD (OR:1.75, OR:1.46, p-values < 0.05). There was no significant difference in tumor histology between both groups (Table 1). Patients with AD were more likely to be diagnosed with stage I lung cancer (36.8% versus 26.9%, p < 0.001) and with low T stage (OR:0.76, p= 0.01), even after controlling for age, sex, race, and histology. Notably, lung cancer patients with AD had a significantly prolonged median overall survival (OS) (7.11 years versus 1.7 years, p < 0.001), independent of stage. Interestingly, lung cancer patients with AD who have received immunotherapy also had a prolonged overall survival (3.8 years versus 2.3 years, p= 0.02).
Conclusion: Patients with rheumatic AD and lung cancer had lower stage disease at diagnosis as compared to lung cancer patients without AD. Patients with AD also had improved OS compared to their counterparts, independent of cancer stage and treatment modalities. Additional studies are needed to investigate tumor size at diagnosis and cancer prognosis within specific subtypes of AD.
.jpg) AD, autoimmune disease; NSCLC, non-small cell lung cancer; SCC, small cell lung cancer; NOS, not otherwise specified
AD, autoimmune disease; NSCLC, non-small cell lung cancer; SCC, small cell lung cancer; NOS, not otherwise specified
*T stage describes the size of the tumor and any spread of cancer into nearby tissue; defined by the eight-edition stage of the American Joint Commission on Cancer (AJCC)
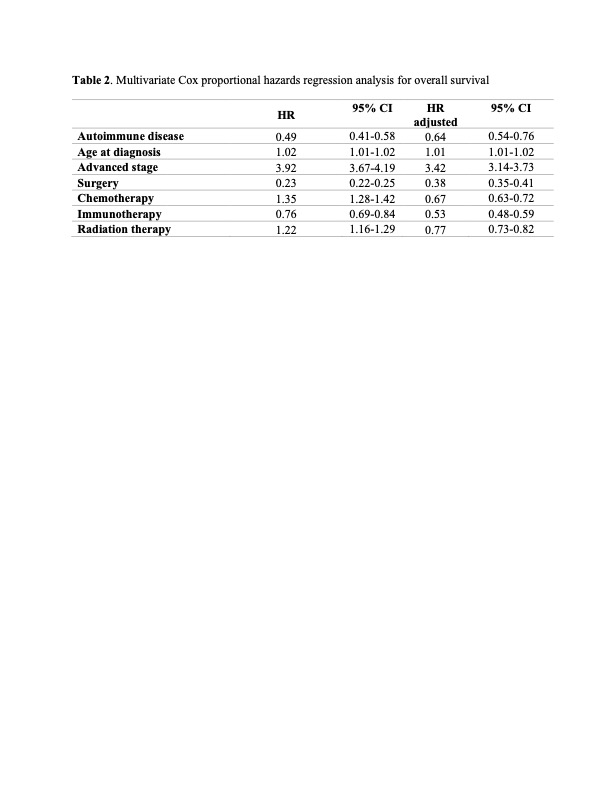 The adjusted OR were controlled for age, stage of the lung cancer diagnosis and the different treatment modalities
The adjusted OR were controlled for age, stage of the lung cancer diagnosis and the different treatment modalities
Disclosures: P. Ghanem, None; J. C. Murray, MJH Life Sciences, Johnson & Johnson; C. Mecoli, Boehringer-Ingelheim; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; B. Levy, AstraZeneca, Pfizer, Eli Liilly, Merck/MSD, Janssen, Miradi, Genentech, Novartis, Daiichi Sankyo, Amgen; K. A. Marrone, Amgen, Mirati, Janssen, Puma, AstraZeneca; D. Ettinger, Beyond Springs was; V. Anagnostou, AstraZeneca, Bristol-Myers Squibb(BMS), US National Institutes of Health, V Foundation, LUNGevity Foundation; V. K. Lam, SeaGen, Bristol-Myers Squibb(BMS), AstraZeneca, Guardant, Takeda; J. R. Brahmer, Bristol-Myers Squibb(BMS), Merck/MSD, Genentech, Janssen, Sanofi, AstraZeneca, Amgen, RAPT Therapeutics; L. C. Cappelli, Bristol-Myers Squibb(BMS).
Background/Purpose: Lung cancer remains the leading cause of cancer-related death. Concomitant autoimmune diseases (AD) can add morbidity and complicate treatment decisions. We evaluated the tumor characteristics at diagnosis and clinical outcomes in lung cancer patients with and without rheumatic AD.
Methods: This is a retrospective cohort study of 10,963 patients with lung cancer from 2004 till 2021, treated at Johns Hopkins Hospital (IRB00276019). Clinical data was extracted from the institutional cancer registry and included demographic features, tumor characteristics, and clinical outcomes. Data on patients' history of 20 autoimmune rheumatic diseases was extracted from the electronic medical record based on ICD10 codes. Chi-square tests and independent t-tests were used to evaluate the association between patient and tumor characteristics and history of AD. To further evaluate the associations between tumor characteristics at diagnosis and AD, we performed multivariate logistic regression accounting for potentially confounding patient characteristics. Kaplan-Meier curves and Cox proportional hazards models were used to compare the overall survival of lung cancer patients with and without AD, adjusting for treatment modalities, age, stage and the treatment modalities (surgery, chemotherapy, immunotherapy, radiation therapy).
Results: AD was documented in 3.6% of lung cancer patients (n=454); the most common AD was rheumatoid arthritis followed by psoriasis/psoriatic arthritis (Figure 1). The age at the time of lung cancer diagnosis was 69 ± 10 and 67 ± 12 years in patients with and without AD, respectively (p= 0.02). Female sex and former smoking status were found to be significantly associated with a history of AD (OR:1.75, OR:1.46, p-values < 0.05). There was no significant difference in tumor histology between both groups (Table 1). Patients with AD were more likely to be diagnosed with stage I lung cancer (36.8% versus 26.9%, p < 0.001) and with low T stage (OR:0.76, p= 0.01), even after controlling for age, sex, race, and histology. Notably, lung cancer patients with AD had a significantly prolonged median overall survival (OS) (7.11 years versus 1.7 years, p < 0.001), independent of stage. Interestingly, lung cancer patients with AD who have received immunotherapy also had a prolonged overall survival (3.8 years versus 2.3 years, p= 0.02).
Conclusion: Patients with rheumatic AD and lung cancer had lower stage disease at diagnosis as compared to lung cancer patients without AD. Patients with AD also had improved OS compared to their counterparts, independent of cancer stage and treatment modalities. Additional studies are needed to investigate tumor size at diagnosis and cancer prognosis within specific subtypes of AD.
.jpg) AD, autoimmune disease; NSCLC, non-small cell lung cancer; SCC, small cell lung cancer; NOS, not otherwise specified
AD, autoimmune disease; NSCLC, non-small cell lung cancer; SCC, small cell lung cancer; NOS, not otherwise specified*T stage describes the size of the tumor and any spread of cancer into nearby tissue; defined by the eight-edition stage of the American Joint Commission on Cancer (AJCC)
 The adjusted OR were controlled for age, stage of the lung cancer diagnosis and the different treatment modalities
The adjusted OR were controlled for age, stage of the lung cancer diagnosis and the different treatment modalities
Disclosures: P. Ghanem, None; J. C. Murray, MJH Life Sciences, Johnson & Johnson; C. Mecoli, Boehringer-Ingelheim; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; B. Levy, AstraZeneca, Pfizer, Eli Liilly, Merck/MSD, Janssen, Miradi, Genentech, Novartis, Daiichi Sankyo, Amgen; K. A. Marrone, Amgen, Mirati, Janssen, Puma, AstraZeneca; D. Ettinger, Beyond Springs was; V. Anagnostou, AstraZeneca, Bristol-Myers Squibb(BMS), US National Institutes of Health, V Foundation, LUNGevity Foundation; V. K. Lam, SeaGen, Bristol-Myers Squibb(BMS), AstraZeneca, Guardant, Takeda; J. R. Brahmer, Bristol-Myers Squibb(BMS), Merck/MSD, Genentech, Janssen, Sanofi, AstraZeneca, Amgen, RAPT Therapeutics; L. C. Cappelli, Bristol-Myers Squibb(BMS).

