Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1894: A Phase 2, 104-Week Study of Repeat Lorecivivint Injections Evaluating Safety, Efficacy, and Bone Health Utilizing Quantitative Computed Tomography (qCT) in Knee Osteoarthritis (OA-06)
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NL
Nancy Lane, MD
University of California
Hillsborough, CA, United States
Abstract Poster Presenter(s)
Yusuf Yazici1, Christopher Swearingen2, Heli Ghandehari3, Jon Britt4, ismail simsek5, Mark Fineman6, Sarah Kennedy2, Jeyanesh Tambiah7 and Nancy Lane8, 1New York University School of Medicine, La Jolla, CA, 2Biosplice Therapeutics, Inc, San Diego, CA, 3Biosplice Therapeutics, Inc., San Diego, CA, 4Biosplice Therapeutics, Inc., Los Angeles, CA, 5Alpine Immunesciences, San Diego, CA, 6Biosplice Therapeutics, San Diego, CA, 7Biosplice Ther Inc., San Diego, CA, 8University of California Davis, Hillsborough, CA
Background/Purpose: Knee osteoarthritis (OA) is a common joint disorder associated with pain, disability, and joint damage. There is a large unmet need for safe and efficacious treatments for treatment of symptoms and structural modification. Lorecivivint (LOR), an intra-articular (IA) CLK/DYRK inhibitor that modulates Wnt and inflammatory pathways, is in development as a potential treatment for knee OA. The primary objective of this trial was to assess the safety and tolerability of repeated 6-month dosing of LOR in a 104-week trial (OA-06, NCT03727022). Additionally, this trial sought to characterize juxta-articular bone health using quantitative computed tomography (qCT) and regional bone health via dual energy x-ray absorptiometry (DXA).
Methods: Participants with ACR-defined clinical and radiographic OA, aged 40-80, and Kellgren-Lawrence (KL) grades 2-3 were randomized 1:1 to receive IA injections of 2 mL 0.07 mg LOR or vehicle PBO at baseline, 24 weeks, 52 weeks, and 72 weeks (4 injections total). The trial was conducted in two 52-week phases, part A (baseline through week 52) and part B (week 53 through week 104), with part A completers invited to part B. General safety was assessed by physical examinations, clinical laboratory tests, collection of adverse events (AEs), and serious AEs (SAEs). Bone safety assessments included qCT (standardized by use of a calibration phantom) to assess BMD in both target and non-target knee, bone and cartilage biomarkers, and DXA to assess spine and hip BMD. Exploratory efficacy was assessed by patient-reported outcomes (PROs). For bone imaging endpoints, change from baseline was estimated using baseline-adjusted ANCOVA.
Results: 101 participants (mean age 60.9±9.1 years, BMI 28.6±3.7 kg/m2, female 59.4%, KL2 52.5%) were enrolled. 77 participants completed part A and 53 completed part B. AE rates were similar between PBO and LOR, and no SAEs were deemed related to treatment. There were no clinical signals for change in bone health, with no fractures, accelerated OA, or osteoporosis observed in LOR or PBO. Observed target knee BMD values as assessed by qCT were similar between LOR and PBO (Figure 1). There were no effects of repeated injection on rates of change in BMDs; the change from baseline in BMD at Week 104 was -7.08 (12.34) mg/cm2 in LOR and -2.95 (8.65) mg/cm2 in PBO, (estimated difference -4.05 [95% CI -11.21, 3.11], not significant). Trends in target knee BMD in those with potentially higher risk of decreasing bone density, female and age [65-80], showed no meaningful differences between LOR and PBO. There were no significant differences in total hip or spine BMD between the LOR and placebo treatment groups.
Potential confounding factors included baseline imbalances in sex (PBO 68.0% vs. LOR 51.0% female), KL grade (PBO 62.0% vs LOR 42.1% KL 2), and site randomization. There were no meaningful differences in PRO changes between LOR and PBO groups. A trial conduct limitation was the small number of qCT-enabled sites available in the US.
Conclusion: The incidence of AEs was similar between treatment groups and not affected by repeated injections of LOR. Multiple injections of LOR over 2 years did not appear to lead to any bone health adverse effects locally around the knee or regionally at spine or hip.
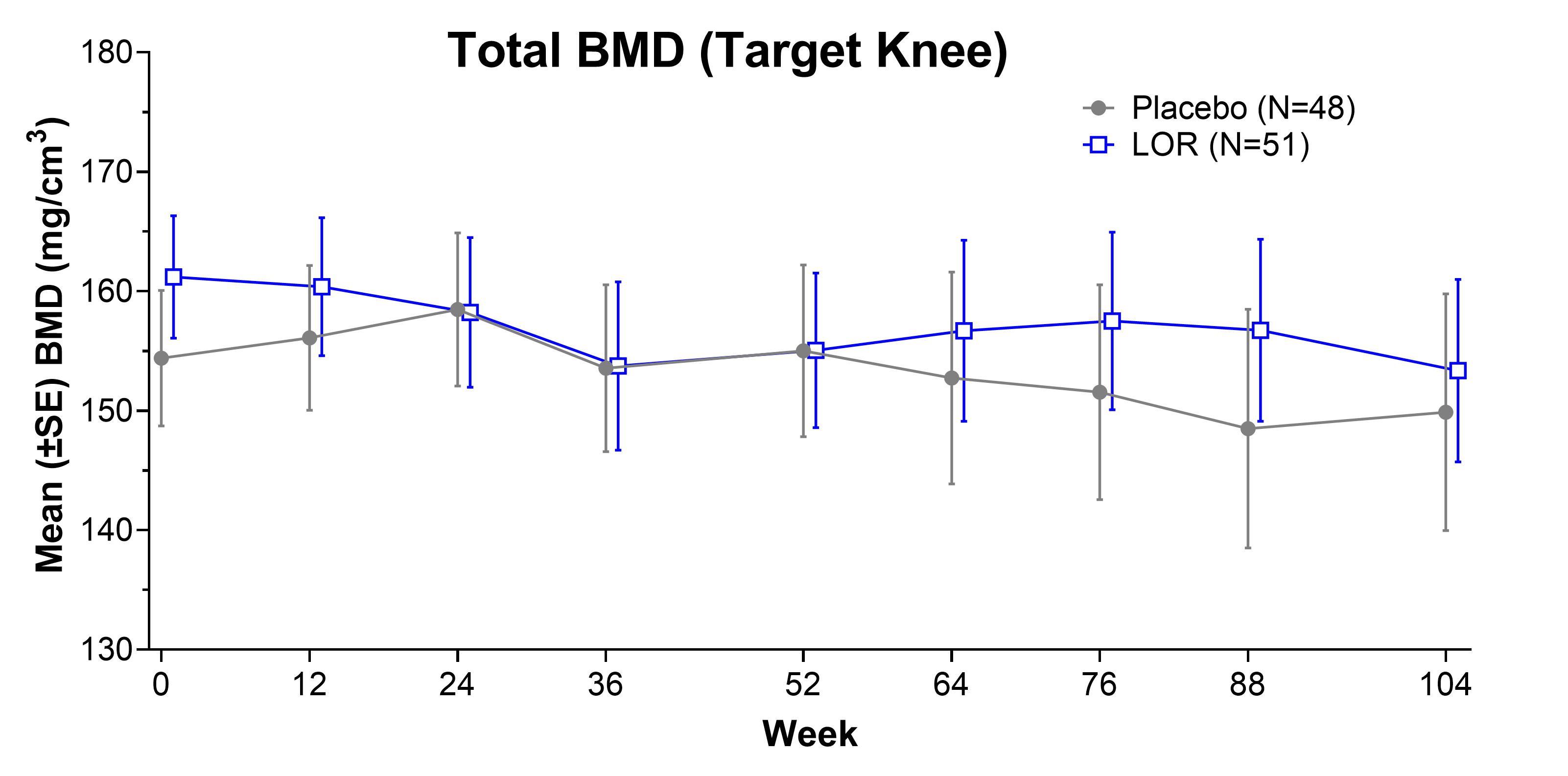 Figure 1. Total Target Knee BMD over 104 weeks
Figure 1. Total Target Knee BMD over 104 weeks
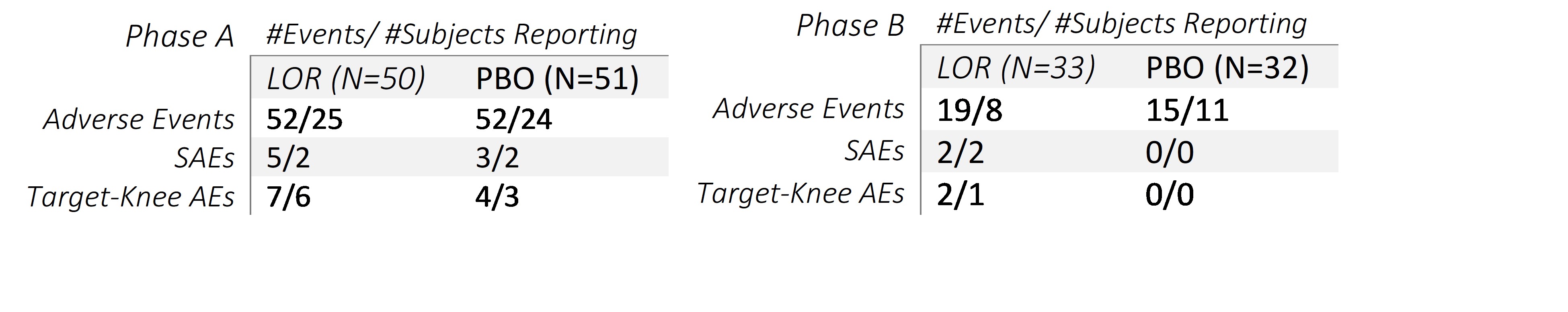 Table 1A and 1B
Table 1A and 1B
Disclosures: Y. Yazici, Amgen, Biosplice; C. Swearingen, Biosplice Therapeutics, Inc; H. Ghandehari, Biosplice Therapeutics, Inc.; J. Britt, Biosplice Therapeutics; i. simsek, Biosplice Inc.; M. Fineman, None; S. Kennedy, Biosplice Therapeutics, Inc; J. Tambiah, Biosplice Therapeutics Inc; N. Lane, Amgen, GlaxoSmithKlein(GSK).
Background/Purpose: Knee osteoarthritis (OA) is a common joint disorder associated with pain, disability, and joint damage. There is a large unmet need for safe and efficacious treatments for treatment of symptoms and structural modification. Lorecivivint (LOR), an intra-articular (IA) CLK/DYRK inhibitor that modulates Wnt and inflammatory pathways, is in development as a potential treatment for knee OA. The primary objective of this trial was to assess the safety and tolerability of repeated 6-month dosing of LOR in a 104-week trial (OA-06, NCT03727022). Additionally, this trial sought to characterize juxta-articular bone health using quantitative computed tomography (qCT) and regional bone health via dual energy x-ray absorptiometry (DXA).
Methods: Participants with ACR-defined clinical and radiographic OA, aged 40-80, and Kellgren-Lawrence (KL) grades 2-3 were randomized 1:1 to receive IA injections of 2 mL 0.07 mg LOR or vehicle PBO at baseline, 24 weeks, 52 weeks, and 72 weeks (4 injections total). The trial was conducted in two 52-week phases, part A (baseline through week 52) and part B (week 53 through week 104), with part A completers invited to part B. General safety was assessed by physical examinations, clinical laboratory tests, collection of adverse events (AEs), and serious AEs (SAEs). Bone safety assessments included qCT (standardized by use of a calibration phantom) to assess BMD in both target and non-target knee, bone and cartilage biomarkers, and DXA to assess spine and hip BMD. Exploratory efficacy was assessed by patient-reported outcomes (PROs). For bone imaging endpoints, change from baseline was estimated using baseline-adjusted ANCOVA.
Results: 101 participants (mean age 60.9±9.1 years, BMI 28.6±3.7 kg/m2, female 59.4%, KL2 52.5%) were enrolled. 77 participants completed part A and 53 completed part B. AE rates were similar between PBO and LOR, and no SAEs were deemed related to treatment. There were no clinical signals for change in bone health, with no fractures, accelerated OA, or osteoporosis observed in LOR or PBO. Observed target knee BMD values as assessed by qCT were similar between LOR and PBO (Figure 1). There were no effects of repeated injection on rates of change in BMDs; the change from baseline in BMD at Week 104 was -7.08 (12.34) mg/cm2 in LOR and -2.95 (8.65) mg/cm2 in PBO, (estimated difference -4.05 [95% CI -11.21, 3.11], not significant). Trends in target knee BMD in those with potentially higher risk of decreasing bone density, female and age [65-80], showed no meaningful differences between LOR and PBO. There were no significant differences in total hip or spine BMD between the LOR and placebo treatment groups.
Potential confounding factors included baseline imbalances in sex (PBO 68.0% vs. LOR 51.0% female), KL grade (PBO 62.0% vs LOR 42.1% KL 2), and site randomization. There were no meaningful differences in PRO changes between LOR and PBO groups. A trial conduct limitation was the small number of qCT-enabled sites available in the US.
Conclusion: The incidence of AEs was similar between treatment groups and not affected by repeated injections of LOR. Multiple injections of LOR over 2 years did not appear to lead to any bone health adverse effects locally around the knee or regionally at spine or hip.
 Figure 1. Total Target Knee BMD over 104 weeks
Figure 1. Total Target Knee BMD over 104 weeks Table 1A and 1B
Table 1A and 1BDisclosures: Y. Yazici, Amgen, Biosplice; C. Swearingen, Biosplice Therapeutics, Inc; H. Ghandehari, Biosplice Therapeutics, Inc.; J. Britt, Biosplice Therapeutics; i. simsek, Biosplice Inc.; M. Fineman, None; S. Kennedy, Biosplice Therapeutics, Inc; J. Tambiah, Biosplice Therapeutics Inc; N. Lane, Amgen, GlaxoSmithKlein(GSK).

