Back
Poster Session D
Immunobiology
Session: (1727–1749) T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
1735: Clonally Expanded Cytotoxic CD8+ T Cells Recognize Citrullinated Antigens in ACPA+ Rheumatoid Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JM
Jae-Seung Moon, PhD
Stanford University
Palo Alto, CA, United States
Abstract Poster Presenter(s)
Jae-Seung Moon1, Shady Younis2, Orr Sharpe2, Navin Rao3, Julie Carman3, Eddie James4, Jane Buckner4, Kevin D Deane5, Michael Holers6, Laura Donlin7, Mark Davis2 and William Robinson8, 1Stanford University, Palo Alto, CA, 2Stanford University, Stanford, CA, 3Janssen Research and Development, LLC, Spring House, PA, 4Benaroya Research Institute at Virginia Mason, Seattle, WA, 5University of Colorado Denver Anschutz Medical Campus, Denver, CO, 6Department of Medicine Division of Rheumatology, Aurora, CO, 7Hospital for Special Surgery, New York, NY, 8Stanford University School of Medicine, Palo Alto, CA
Background/Purpose: Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with MHC polymorphisms. The shared epitope polymorphism in MHC class II genes is by far the strongest genetic association with ACPA+ RA, and the shared epitope polymorphisms have been demonstrated to facilitate the binding and presentation of citrullinated peptides. MHC class I gene polymorphisms are also associated with the susceptibility to seropositive RA. To further investigate the potential role of MHC class I polymorphisms in the pathogenesis of RA, we characterized CD8+ T cell phenotypes and clonality in ACPA+ RA blood using flow cytometric and single cell RNA and TCR sequencing analysis. We also performed in vitro assays to characterize the antigen specificity and functional properties of clonally expanded CD8+ T cells in ACPA+ RA blood.
Methods: We used flow cytometric and single-cell RNA profiling analyses including VDJ sequencing to analyze the effector phenotypes and clonal expansion of CD8+ T cells in ACPA+ RA blood. To investigate the antigen targets of the expanded CD8+ T cells in ACPA+ RA, we performed in vitro protein stimulation in ACPA+ RA peripheral blood mononuclear cells (PBMCs) or whole blood with candidate citrullinated RA antigens. To assess the cytotoxic potential of citrullinated antigen-activated cytotoxic of CD8+ T cells, we used an in vitro MHC-independent cytotoxicity assay.
Results: We identified GZMB+CD8+ subpopulations in ACPA+ RA blood containing large clonal lineage expansions expressing cytotoxic and tissue homing transcriptional programs, and a GZMK+CD8+ memory subpopulation comprised of smaller clonal expansions expressing effector T cell transcriptional programs. We demonstrated citrullinated RA autoantigens presented by MHC class I activate ACPA+ RA blood-derived GZMB+CD8+ T cells to expand, express cytotoxic mediators, and mediate killing of target cells. We also demonstrated that these activated GZMB+CD8+ T cells are present in RA synovium.
Conclusion: Our findings demonstrated that clonally expanded GZMB+ CD8+ T cells are present in ACPA+ RA blood. We further demonstrated that these clonally expanded cytotoxic CD8+ T cells are specific for citrullinated antigens and can mediate cytolytic cell killing. Our findings suggest that cytotoxic CD8+ T cells targeting citrullinated antigens could contribute to synovitis and joint tissue destruction in ACPA+ RA.
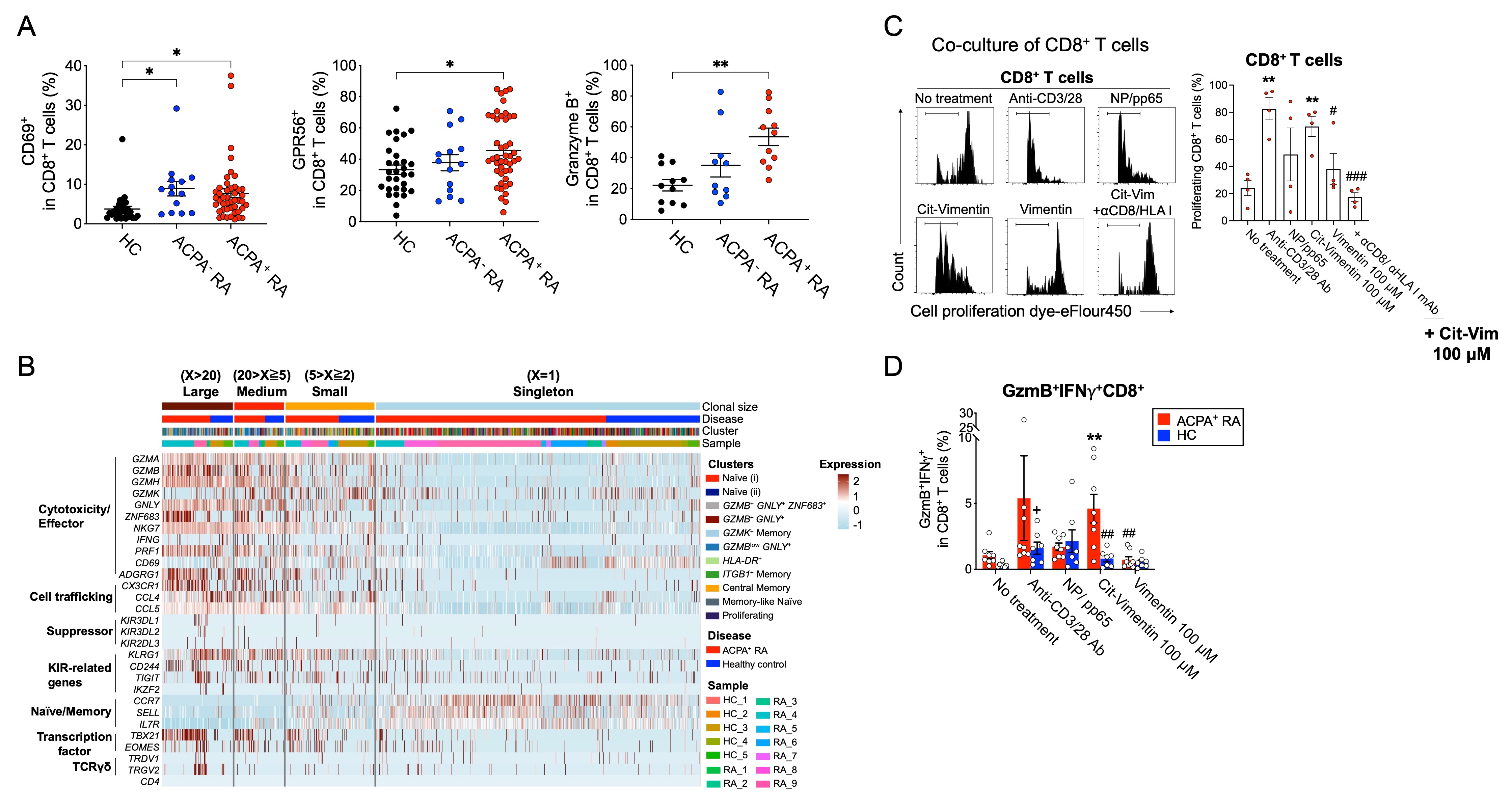 Clonally expanded cytotoxic CD8+ T cells reactive to citrullinated antigens are present in ACPA+ RA blood. (A) Percentage of CD69, GPR56 and granzyme B-expressing CD8+ T cells in healthy controls (HC), ACPA- or ACPA+ RA PBMCs. (B) Phenotypic analysis of clonally expanded CD8+ T cells in ACPA+ RA and HC. Heatmap of known gene markers provides the gene expression patterns of clonally expanded cells for: cytotoxicity/Effector, cell trafficking, suppressor, and naïve/memory subsets. (C) Native vimentin or citrullinated vimentin pulsed monocyte-derived dendritic cells were cocultured with Cell Proliferation Dye eF450-labeled CD8+ T cells isolated from ACPA+ RA PBMCs with or without anti-CD8/HLA class I-blocking antibody for 10 days. Proliferation ability of T cells was determined by flow cytometry. (D) Percentage of GzmB+IFNg+ population in CD8+ T cells from ACPA+ RA or HC fresh whole blood stimulation using native or citrullinated vimentin.
Clonally expanded cytotoxic CD8+ T cells reactive to citrullinated antigens are present in ACPA+ RA blood. (A) Percentage of CD69, GPR56 and granzyme B-expressing CD8+ T cells in healthy controls (HC), ACPA- or ACPA+ RA PBMCs. (B) Phenotypic analysis of clonally expanded CD8+ T cells in ACPA+ RA and HC. Heatmap of known gene markers provides the gene expression patterns of clonally expanded cells for: cytotoxicity/Effector, cell trafficking, suppressor, and naïve/memory subsets. (C) Native vimentin or citrullinated vimentin pulsed monocyte-derived dendritic cells were cocultured with Cell Proliferation Dye eF450-labeled CD8+ T cells isolated from ACPA+ RA PBMCs with or without anti-CD8/HLA class I-blocking antibody for 10 days. Proliferation ability of T cells was determined by flow cytometry. (D) Percentage of GzmB+IFNg+ population in CD8+ T cells from ACPA+ RA or HC fresh whole blood stimulation using native or citrullinated vimentin.
Disclosures: J. Moon, None; S. Younis, None; O. Sharpe, None; N. Rao, Janssen; J. Carman, Janssen; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; K. Deane, Werfen; M. Holers, Celgene, Bristol-Myers Squibb(BMS), Janssen; L. Donlin, None; M. Davis, None; W. Robinson, None.
Background/Purpose: Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with MHC polymorphisms. The shared epitope polymorphism in MHC class II genes is by far the strongest genetic association with ACPA+ RA, and the shared epitope polymorphisms have been demonstrated to facilitate the binding and presentation of citrullinated peptides. MHC class I gene polymorphisms are also associated with the susceptibility to seropositive RA. To further investigate the potential role of MHC class I polymorphisms in the pathogenesis of RA, we characterized CD8+ T cell phenotypes and clonality in ACPA+ RA blood using flow cytometric and single cell RNA and TCR sequencing analysis. We also performed in vitro assays to characterize the antigen specificity and functional properties of clonally expanded CD8+ T cells in ACPA+ RA blood.
Methods: We used flow cytometric and single-cell RNA profiling analyses including VDJ sequencing to analyze the effector phenotypes and clonal expansion of CD8+ T cells in ACPA+ RA blood. To investigate the antigen targets of the expanded CD8+ T cells in ACPA+ RA, we performed in vitro protein stimulation in ACPA+ RA peripheral blood mononuclear cells (PBMCs) or whole blood with candidate citrullinated RA antigens. To assess the cytotoxic potential of citrullinated antigen-activated cytotoxic of CD8+ T cells, we used an in vitro MHC-independent cytotoxicity assay.
Results: We identified GZMB+CD8+ subpopulations in ACPA+ RA blood containing large clonal lineage expansions expressing cytotoxic and tissue homing transcriptional programs, and a GZMK+CD8+ memory subpopulation comprised of smaller clonal expansions expressing effector T cell transcriptional programs. We demonstrated citrullinated RA autoantigens presented by MHC class I activate ACPA+ RA blood-derived GZMB+CD8+ T cells to expand, express cytotoxic mediators, and mediate killing of target cells. We also demonstrated that these activated GZMB+CD8+ T cells are present in RA synovium.
Conclusion: Our findings demonstrated that clonally expanded GZMB+ CD8+ T cells are present in ACPA+ RA blood. We further demonstrated that these clonally expanded cytotoxic CD8+ T cells are specific for citrullinated antigens and can mediate cytolytic cell killing. Our findings suggest that cytotoxic CD8+ T cells targeting citrullinated antigens could contribute to synovitis and joint tissue destruction in ACPA+ RA.
 Clonally expanded cytotoxic CD8+ T cells reactive to citrullinated antigens are present in ACPA+ RA blood. (A) Percentage of CD69, GPR56 and granzyme B-expressing CD8+ T cells in healthy controls (HC), ACPA- or ACPA+ RA PBMCs. (B) Phenotypic analysis of clonally expanded CD8+ T cells in ACPA+ RA and HC. Heatmap of known gene markers provides the gene expression patterns of clonally expanded cells for: cytotoxicity/Effector, cell trafficking, suppressor, and naïve/memory subsets. (C) Native vimentin or citrullinated vimentin pulsed monocyte-derived dendritic cells were cocultured with Cell Proliferation Dye eF450-labeled CD8+ T cells isolated from ACPA+ RA PBMCs with or without anti-CD8/HLA class I-blocking antibody for 10 days. Proliferation ability of T cells was determined by flow cytometry. (D) Percentage of GzmB+IFNg+ population in CD8+ T cells from ACPA+ RA or HC fresh whole blood stimulation using native or citrullinated vimentin.
Clonally expanded cytotoxic CD8+ T cells reactive to citrullinated antigens are present in ACPA+ RA blood. (A) Percentage of CD69, GPR56 and granzyme B-expressing CD8+ T cells in healthy controls (HC), ACPA- or ACPA+ RA PBMCs. (B) Phenotypic analysis of clonally expanded CD8+ T cells in ACPA+ RA and HC. Heatmap of known gene markers provides the gene expression patterns of clonally expanded cells for: cytotoxicity/Effector, cell trafficking, suppressor, and naïve/memory subsets. (C) Native vimentin or citrullinated vimentin pulsed monocyte-derived dendritic cells were cocultured with Cell Proliferation Dye eF450-labeled CD8+ T cells isolated from ACPA+ RA PBMCs with or without anti-CD8/HLA class I-blocking antibody for 10 days. Proliferation ability of T cells was determined by flow cytometry. (D) Percentage of GzmB+IFNg+ population in CD8+ T cells from ACPA+ RA or HC fresh whole blood stimulation using native or citrullinated vimentin.Disclosures: J. Moon, None; S. Younis, None; O. Sharpe, None; N. Rao, Janssen; J. Carman, Janssen; E. James, Janssen, Provention Bio, Bristol-Myers Squibb(BMS), Novartis; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; K. Deane, Werfen; M. Holers, Celgene, Bristol-Myers Squibb(BMS), Janssen; L. Donlin, None; M. Davis, None; W. Robinson, None.

