Back
Poster Session D
Session: (1980–2016) RA – Treatment Poster IV
1992: Burden of Pain for Patients in the CorEvitasTM Rheumatoid Arthritis Registry
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Joshua Baker, MD, MS
University of Pennsylvania
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Joshua Baker1, J Morel Symons2, Jud C Janak3, Page Moore3, Elizabeth Kohl3, Bernice Gershenson3, Oksana Pugach3, Dave Webb4, Alan A Martin4, Didier Saurigny5 and Marguerite Bracher5, 1University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 2GlaxoSmithKline, Collegeville, PA, 3CorEvitas, LLC, Waltham, MA, 4GlaxoSmithKline, Brentford, United Kingdom, 5GlaxoSmithKline, Stevenage, United Kingdom
Background/Purpose: Pain is a hallmark symptom of RA that impacts patients' quality of life and informs therapeutic decisions that aim to reduce joint inflammation and associated pain. Even for patients on biologic/targeted synthetic (b/ts)DMARDs, pain can persist, and it is difficult to identify who will experience this persistent pain. The aim of this study was to describe baseline clinical and non-clinical characteristics of patients with RA initiating b/tsDMARDs, stratified by pain improvement or persistence.
Methods: Patients in the CorEvitas RA Registry with the following criteria were included: initiated a b/tsDMARD within 1 month of a CorEvitas visit from 1/1/2015 to 1/1/2020; had clinically meaningful baseline pain (Pain visual analog scale [VAS; 0–100 mm] >20); and a second Pain VAS at 6 (3–9) months follow-up. Patients were stratified by line of targeted therapy and baseline Pain VAS: mild (21–44 mm), moderate (45–74 mm), severe (≥75 mm). Improved pain was defined as a Pain VAS decrease from baseline of ≥70%, ≥50%, or ≥30% at 6 months. Persistent pain was defined as worsening or failure to improve. Within line of therapy and baseline pain categories, mean/proportional differences and 95% confidence intervals (CI) for patient characteristics were calculated for the three criteria of improved vs persistent pain.
Results: A total of 3287 patients were included: 26% mild, 40% moderate, 34% severe pain. Across all lines of therapy and baseline pain categories, ≥50% of patients reported persistent pain at 6 months (Figure). Among second/third+ b/tsDMARD initiators (n=2385), ~75% reported persistent pain (improvement < 50%). Among b/tsDMARD-naïve patients with severe baseline pain, more patients with persistent pain (improvement < 30%; n=140) vs improved pain (improvement ≥30%; n=140) had the following characteristics: Medicaid insurance (19% vs 6%; Δ=13%; 95% CI 5%–20%), current smoker (29% vs 16%; Δ=13%; 95% CI 3%–23%), history of fibromyalgia (20% vs 9%; Δ=11%; 95% CI 3%–19%), difficulty sleeping (67% vs 45%; Δ=22%; 95% CI 11%–33%), current opioid use (43% vs 30%; Δ=13%; 95% CI 2%–25%), and concomitant conventional DMARD therapy (43% vs 29%; Δ=14%; 95% CI 3%–25%). Among patients on second line of therapy with severe baseline pain, more patients with persistent pain (improvement < 30%; n=242) vs improved pain (improvement ≥30%; n=151) were more likely to report: history of hypertension (40% vs 29%; Δ=11%; 95% CI 1%–20%), history of fibromyalgia (21% vs 9%; Δ=12%; 95% CI 5%–18%), difficulty sleeping (52% vs 40%; Δ=12%; 95% CI 2%–22%), and current opioid use (53% vs 39%; Δ=14%; 95% CI 4%–25%).
Conclusion: This study highlights the persistent burden of pain for many patients with RA initiating b/tsDMARDs, regardless of baseline pain severity or line of therapy. Multidisciplinary treatment approaches to effectively manage RA pain are critical and targeted interventions with novel b/tsDMARDs remain promising. As persistent pain is associated with chronic opioid use, further research to identify risk factors may reduce opioid use for this population.
This GSK-funded study (213726) was sponsored by CorEvitas, LLC, with editorial support by Fishawack Indicia Ltd, funded by GSK.
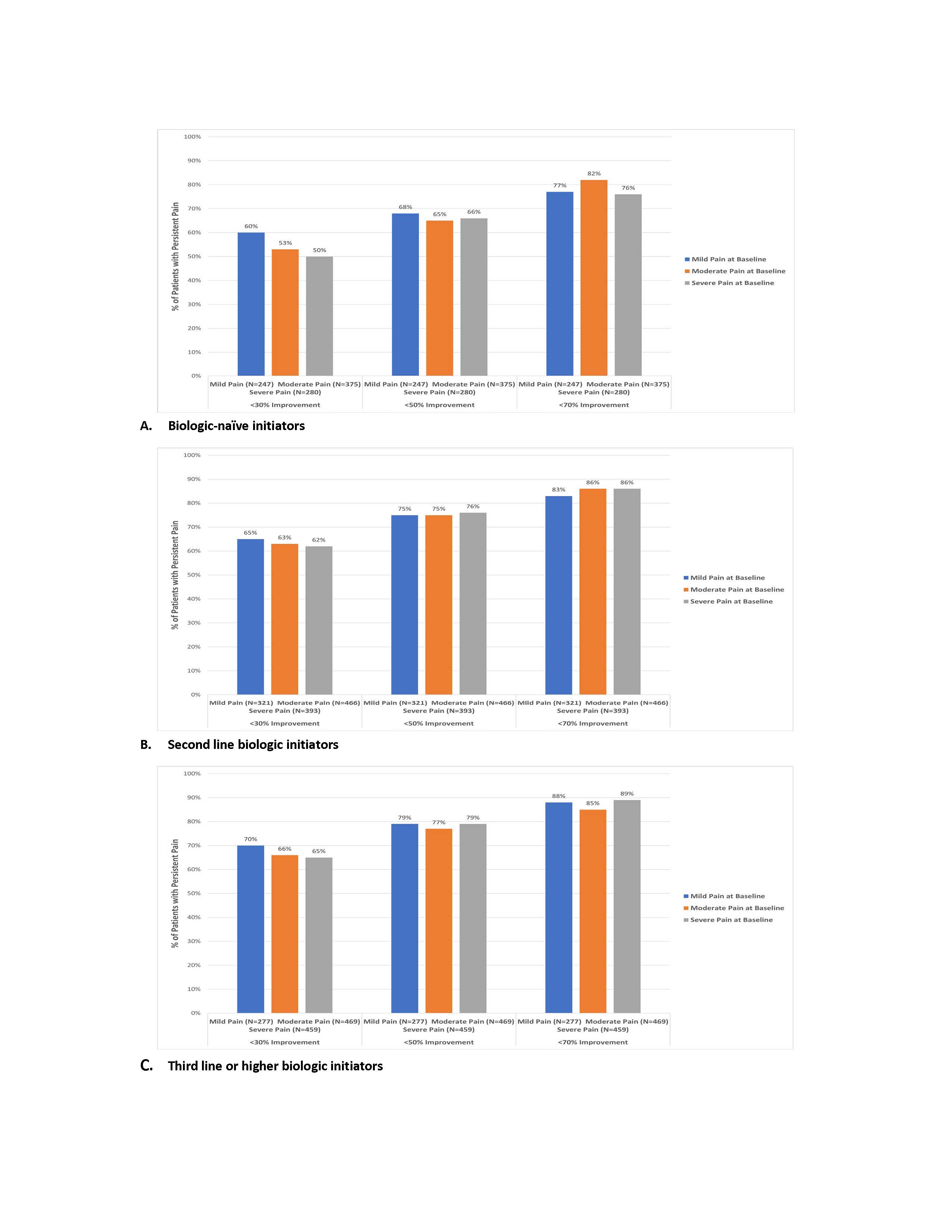 Figure. Percent of patients with persistent pain failing to achieve ≥30%, ≥50%, and ≥70% improvement in pain 6 months post-initiation by line of therapy, stratified by baseline pain category
Figure. Percent of patients with persistent pain failing to achieve ≥30%, ≥50%, and ≥70% improvement in pain 6 months post-initiation by line of therapy, stratified by baseline pain category
Disclosures: J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Symons, GlaxoSmithKline (GSK); J. Janak, CorEvitas LLC; P. Moore, None; E. Kohl, CorEvitas LLC; B. Gershenson, None; O. Pugach, CorEvitas LLC; D. Webb, GlaxoSmithKline (GSK); A. Martin, GlaxoSmithKline (GSK); D. Saurigny, GlaxoSmithKline (GSK); M. Bracher, GlaxoSmithKline (GSK).
Background/Purpose: Pain is a hallmark symptom of RA that impacts patients' quality of life and informs therapeutic decisions that aim to reduce joint inflammation and associated pain. Even for patients on biologic/targeted synthetic (b/ts)DMARDs, pain can persist, and it is difficult to identify who will experience this persistent pain. The aim of this study was to describe baseline clinical and non-clinical characteristics of patients with RA initiating b/tsDMARDs, stratified by pain improvement or persistence.
Methods: Patients in the CorEvitas RA Registry with the following criteria were included: initiated a b/tsDMARD within 1 month of a CorEvitas visit from 1/1/2015 to 1/1/2020; had clinically meaningful baseline pain (Pain visual analog scale [VAS; 0–100 mm] >20); and a second Pain VAS at 6 (3–9) months follow-up. Patients were stratified by line of targeted therapy and baseline Pain VAS: mild (21–44 mm), moderate (45–74 mm), severe (≥75 mm). Improved pain was defined as a Pain VAS decrease from baseline of ≥70%, ≥50%, or ≥30% at 6 months. Persistent pain was defined as worsening or failure to improve. Within line of therapy and baseline pain categories, mean/proportional differences and 95% confidence intervals (CI) for patient characteristics were calculated for the three criteria of improved vs persistent pain.
Results: A total of 3287 patients were included: 26% mild, 40% moderate, 34% severe pain. Across all lines of therapy and baseline pain categories, ≥50% of patients reported persistent pain at 6 months (Figure). Among second/third+ b/tsDMARD initiators (n=2385), ~75% reported persistent pain (improvement < 50%). Among b/tsDMARD-naïve patients with severe baseline pain, more patients with persistent pain (improvement < 30%; n=140) vs improved pain (improvement ≥30%; n=140) had the following characteristics: Medicaid insurance (19% vs 6%; Δ=13%; 95% CI 5%–20%), current smoker (29% vs 16%; Δ=13%; 95% CI 3%–23%), history of fibromyalgia (20% vs 9%; Δ=11%; 95% CI 3%–19%), difficulty sleeping (67% vs 45%; Δ=22%; 95% CI 11%–33%), current opioid use (43% vs 30%; Δ=13%; 95% CI 2%–25%), and concomitant conventional DMARD therapy (43% vs 29%; Δ=14%; 95% CI 3%–25%). Among patients on second line of therapy with severe baseline pain, more patients with persistent pain (improvement < 30%; n=242) vs improved pain (improvement ≥30%; n=151) were more likely to report: history of hypertension (40% vs 29%; Δ=11%; 95% CI 1%–20%), history of fibromyalgia (21% vs 9%; Δ=12%; 95% CI 5%–18%), difficulty sleeping (52% vs 40%; Δ=12%; 95% CI 2%–22%), and current opioid use (53% vs 39%; Δ=14%; 95% CI 4%–25%).
Conclusion: This study highlights the persistent burden of pain for many patients with RA initiating b/tsDMARDs, regardless of baseline pain severity or line of therapy. Multidisciplinary treatment approaches to effectively manage RA pain are critical and targeted interventions with novel b/tsDMARDs remain promising. As persistent pain is associated with chronic opioid use, further research to identify risk factors may reduce opioid use for this population.
This GSK-funded study (213726) was sponsored by CorEvitas, LLC, with editorial support by Fishawack Indicia Ltd, funded by GSK.
 Figure. Percent of patients with persistent pain failing to achieve ≥30%, ≥50%, and ≥70% improvement in pain 6 months post-initiation by line of therapy, stratified by baseline pain category
Figure. Percent of patients with persistent pain failing to achieve ≥30%, ≥50%, and ≥70% improvement in pain 6 months post-initiation by line of therapy, stratified by baseline pain category Disclosures: J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Symons, GlaxoSmithKline (GSK); J. Janak, CorEvitas LLC; P. Moore, None; E. Kohl, CorEvitas LLC; B. Gershenson, None; O. Pugach, CorEvitas LLC; D. Webb, GlaxoSmithKline (GSK); A. Martin, GlaxoSmithKline (GSK); D. Saurigny, GlaxoSmithKline (GSK); M. Bracher, GlaxoSmithKline (GSK).

