Back
Plenary Session
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: Plenary I (0001–0005)
0004: mTORC1 Drives the Spectrum of Pathology in Systemic JIA and Macrophage Activation Syndrome
Saturday, November 12, 2022
12:30 PM – 12:40 PM Eastern Time
Location: Exhibit Hall A
- PL
Pui Lee, MD, PhD
Boston Children's Hospital
Newton, MA, United States
Presenting Author(s)
zhengping huang1, xiaomeng You2, Liang Chen3, Yan Du3, Kailey Brodeur3, Qiang Wang3, David Sykes4, Margaret Chang5, Julia Charles2, Peter Nigrovic5 and Pui Lee5, 1Guangdong Second Provincial Hospital, Guangzhou, China, 2Brigham and Women's Hospital, Boston, MA, 3Boston Children's Hospital, Boston, MA, 4Massachusetts General Hospital, Boston, MA, 5Division of Immunology, Boston Children's Hospital, Boston, MA
Background/Purpose: Systemic juvenile idiopathic arthritis (sJIA) is a severe inflammatory syndrome characterized by fever, skin rash and arthritis. A subset of patients with sJIA develop macrophage activation syndrome (MAS), a potentially fatal complication of immune dysregulation resulting in cytokine storm. We aim to understand the pathologic link between sJIA and MAS.
Methods: We modeled the biology of sJIA using mice deficient in IL1 receptor antagonist (Il1rn-/-) and examined the impact of mTORC1 (mechanistic target of rapamycin complex 1) inhibition and phagocyte depletion. We analyzed available transcriptomic datasets from healthy controls and patients with sJIA. We studied the impact of mTORC1 inhibition on CpG-induced MAS and investigated features of MAS in a murine model of excessive mTORC1 activation.
Results: Il1rn-/- mice recapitulated key features of sJIA including arthritis, systemic inflammation, myeloid cell expansion and splenomegaly. Single-cell RNA sequencing and phospho-flow cytometry analysis of peripheral blood leukocytes from these mice showed preferential activation of the mTORC1 pathway in monocytes. mTORC1 inhibition using rapamycin or monocyte depletion using clodronate liposomes both attenuated disease severity in Il1rn-/- mice (Figure 1). Published transcriptomic datasets from studies on sJIA revealed an mTORC1 gene signature that correlates with disease activity and treatment response. Interestingly, unrestricted activation of mTORC1 by inducible loss of Tsc2 in mice was sufficient to trigger an sJIA-like syndrome, including both inflammatory arthritis and MAS with hemophagocytosis, a cellular manifestation that could be reproduced in human monocytes by CRISPR/Cas-mediated deletion of TSC2 (Figure 2). Consistent with this observation, hemophagocytic histiocytes from patients with MAS displayed prominent mTORC1 activity. Finally, rapamycin treatment prevented hemophagocytosis in Tsc2-inducible KO mice and also attenuated the disease severity of CpG-induced MAS in wildtype C57BL6 mice.
Conclusion: Our study identifies mTORC1 as a driver of inflammation that connects the pathogenesis of sJIA and MAS.
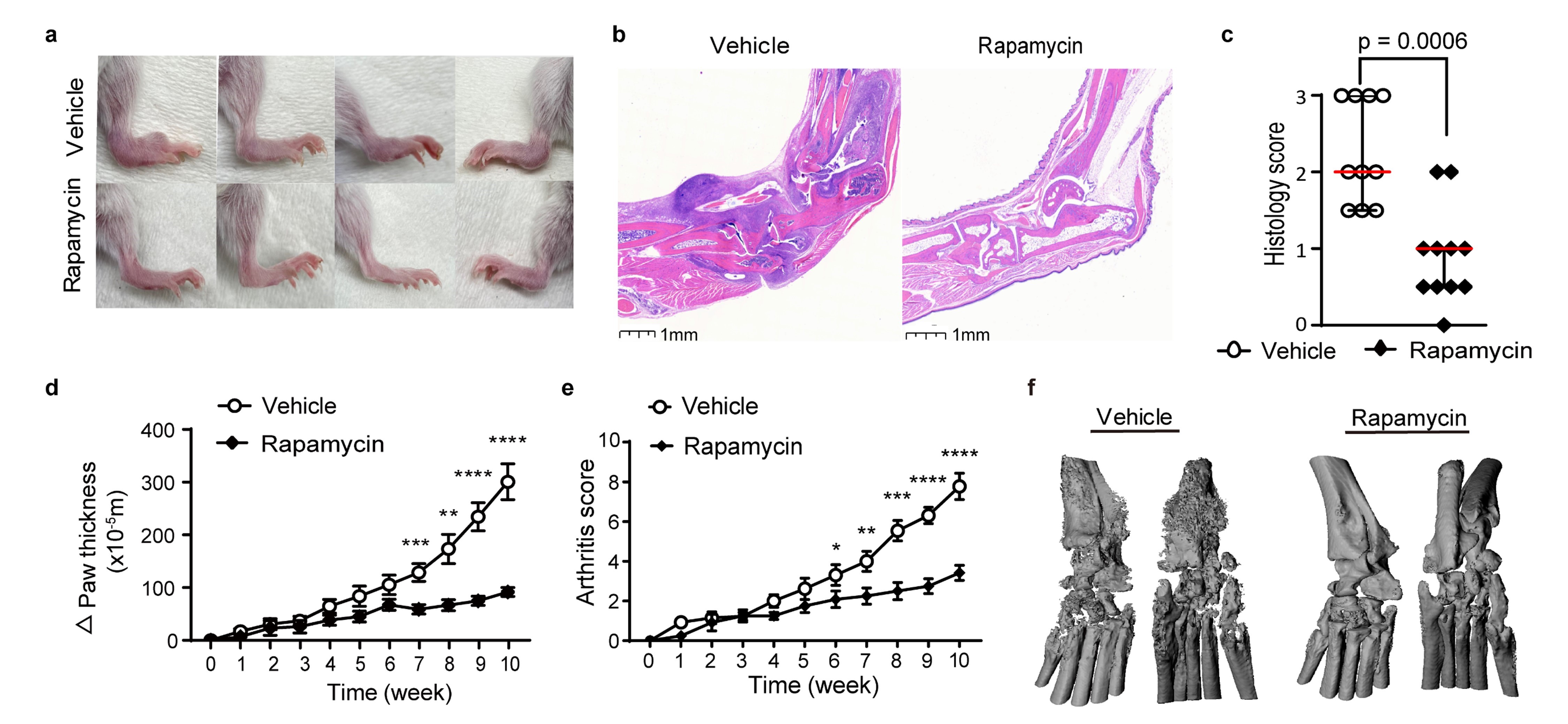 Figure 1. Rapamycin treatment reduces arthritis severity and bone erosion in Il1rn-/- mice.
Figure 1. Rapamycin treatment reduces arthritis severity and bone erosion in Il1rn-/- mice.
a) Representative depiction of ankle inflammation, b) hematoxylin and eosin staining of ankle sections, c) histologic scores of ankle arthritis, d) ankle and wrist thickness, e) composite arthritis score and f) micro-CT evaluation of joint erosion in Il1rn-/- mice treated with rapamycin or vehicle for 10 weeks (n = 10-14 per group). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
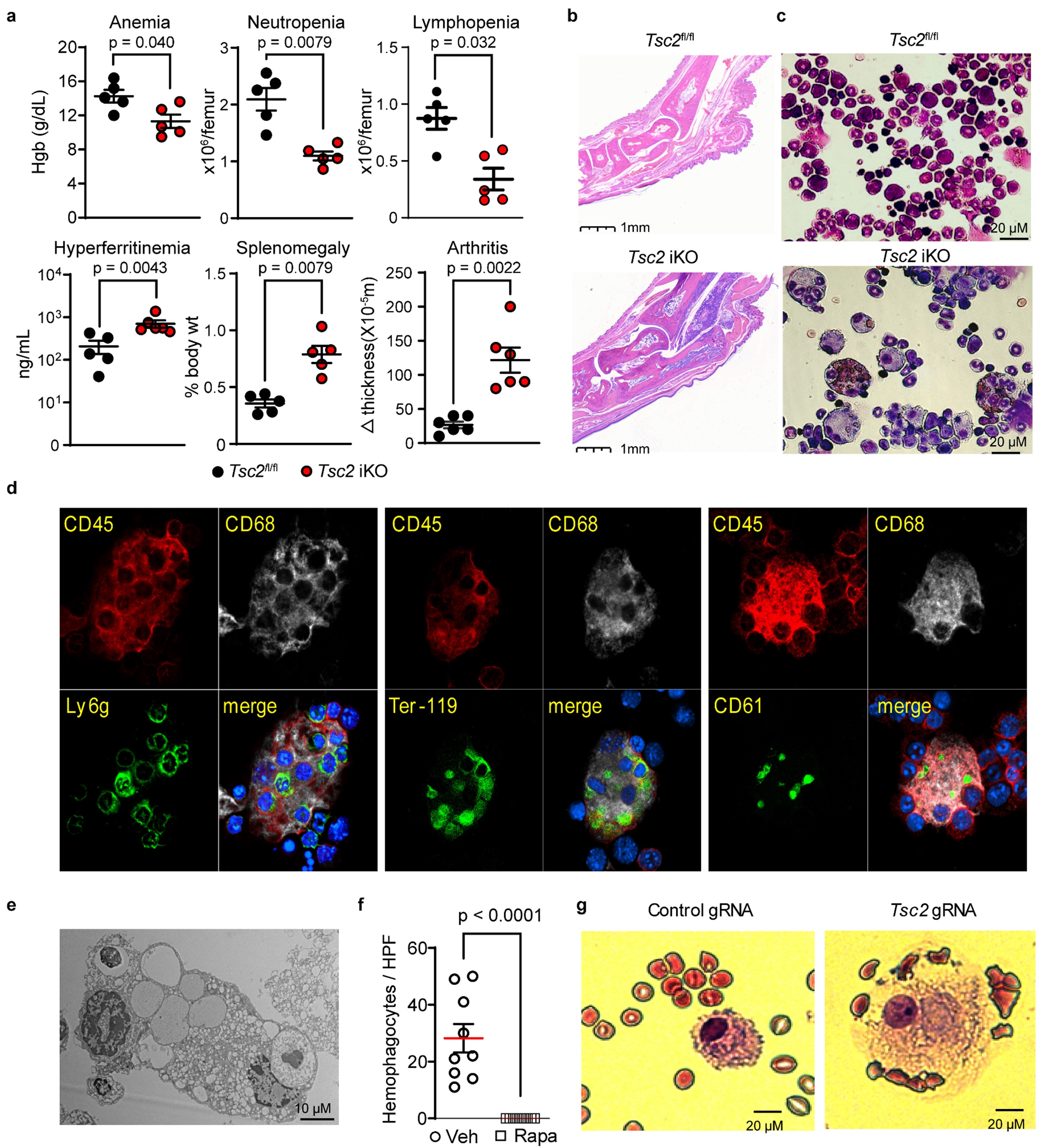 Figure 2. Unrestricted mTORC1 activation drives the development of hemophagocytosis and MAS. a) Hemoglobin levels, bone marrow cell count, plasma ferritin levels, spleen weight, and change in ankle joint thickness and b) representative ankle pathology (H&E stain) in Tsc2 iKO mice and control mice (n = 5-6 per group). c) Wright-Giemsa staining of bone marrow leukocytes from Tsc2 iKO and control mice. d) Confocal microscopy and e) electron microscopy of hemophagocytes from the bone marrow of Tsc2 iKO mice. f) Quantification of bone marrow hemophagocytes in Tsc2 iKO mice treated with rapamycin or vehicle control (3 slides analyzed for each mouse; n = 3 per group). Analyses were performed 3 weeks after poly I:C treatment to induce Tsc2 deletion. g) Wright-Giemsa staining of human monocytes with targeted disruption of Tsc2 by CRISPR/Cas9 cultured with M-CSF for 14 days.
Figure 2. Unrestricted mTORC1 activation drives the development of hemophagocytosis and MAS. a) Hemoglobin levels, bone marrow cell count, plasma ferritin levels, spleen weight, and change in ankle joint thickness and b) representative ankle pathology (H&E stain) in Tsc2 iKO mice and control mice (n = 5-6 per group). c) Wright-Giemsa staining of bone marrow leukocytes from Tsc2 iKO and control mice. d) Confocal microscopy and e) electron microscopy of hemophagocytes from the bone marrow of Tsc2 iKO mice. f) Quantification of bone marrow hemophagocytes in Tsc2 iKO mice treated with rapamycin or vehicle control (3 slides analyzed for each mouse; n = 3 per group). Analyses were performed 3 weeks after poly I:C treatment to induce Tsc2 deletion. g) Wright-Giemsa staining of human monocytes with targeted disruption of Tsc2 by CRISPR/Cas9 cultured with M-CSF for 14 days.
Disclosures: z. huang, None; x. You, None; L. Chen, None; Y. Du, None; K. Brodeur, None; Q. Wang, None; D. Sykes, Clear Creek Bio; M. Chang, None; J. Charles, None; P. Nigrovic, Bristol-Myers Squibb(BMS), Exo Therapeutics, Miach Orthopedics, Pfizer, Novartis, UpToDate, Cerecor, Brickell Bio; P. Lee, None.
Background/Purpose: Systemic juvenile idiopathic arthritis (sJIA) is a severe inflammatory syndrome characterized by fever, skin rash and arthritis. A subset of patients with sJIA develop macrophage activation syndrome (MAS), a potentially fatal complication of immune dysregulation resulting in cytokine storm. We aim to understand the pathologic link between sJIA and MAS.
Methods: We modeled the biology of sJIA using mice deficient in IL1 receptor antagonist (Il1rn-/-) and examined the impact of mTORC1 (mechanistic target of rapamycin complex 1) inhibition and phagocyte depletion. We analyzed available transcriptomic datasets from healthy controls and patients with sJIA. We studied the impact of mTORC1 inhibition on CpG-induced MAS and investigated features of MAS in a murine model of excessive mTORC1 activation.
Results: Il1rn-/- mice recapitulated key features of sJIA including arthritis, systemic inflammation, myeloid cell expansion and splenomegaly. Single-cell RNA sequencing and phospho-flow cytometry analysis of peripheral blood leukocytes from these mice showed preferential activation of the mTORC1 pathway in monocytes. mTORC1 inhibition using rapamycin or monocyte depletion using clodronate liposomes both attenuated disease severity in Il1rn-/- mice (Figure 1). Published transcriptomic datasets from studies on sJIA revealed an mTORC1 gene signature that correlates with disease activity and treatment response. Interestingly, unrestricted activation of mTORC1 by inducible loss of Tsc2 in mice was sufficient to trigger an sJIA-like syndrome, including both inflammatory arthritis and MAS with hemophagocytosis, a cellular manifestation that could be reproduced in human monocytes by CRISPR/Cas-mediated deletion of TSC2 (Figure 2). Consistent with this observation, hemophagocytic histiocytes from patients with MAS displayed prominent mTORC1 activity. Finally, rapamycin treatment prevented hemophagocytosis in Tsc2-inducible KO mice and also attenuated the disease severity of CpG-induced MAS in wildtype C57BL6 mice.
Conclusion: Our study identifies mTORC1 as a driver of inflammation that connects the pathogenesis of sJIA and MAS.
 Figure 1. Rapamycin treatment reduces arthritis severity and bone erosion in Il1rn-/- mice.
Figure 1. Rapamycin treatment reduces arthritis severity and bone erosion in Il1rn-/- mice. a) Representative depiction of ankle inflammation, b) hematoxylin and eosin staining of ankle sections, c) histologic scores of ankle arthritis, d) ankle and wrist thickness, e) composite arthritis score and f) micro-CT evaluation of joint erosion in Il1rn-/- mice treated with rapamycin or vehicle for 10 weeks (n = 10-14 per group). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
 Figure 2. Unrestricted mTORC1 activation drives the development of hemophagocytosis and MAS. a) Hemoglobin levels, bone marrow cell count, plasma ferritin levels, spleen weight, and change in ankle joint thickness and b) representative ankle pathology (H&E stain) in Tsc2 iKO mice and control mice (n = 5-6 per group). c) Wright-Giemsa staining of bone marrow leukocytes from Tsc2 iKO and control mice. d) Confocal microscopy and e) electron microscopy of hemophagocytes from the bone marrow of Tsc2 iKO mice. f) Quantification of bone marrow hemophagocytes in Tsc2 iKO mice treated with rapamycin or vehicle control (3 slides analyzed for each mouse; n = 3 per group). Analyses were performed 3 weeks after poly I:C treatment to induce Tsc2 deletion. g) Wright-Giemsa staining of human monocytes with targeted disruption of Tsc2 by CRISPR/Cas9 cultured with M-CSF for 14 days.
Figure 2. Unrestricted mTORC1 activation drives the development of hemophagocytosis and MAS. a) Hemoglobin levels, bone marrow cell count, plasma ferritin levels, spleen weight, and change in ankle joint thickness and b) representative ankle pathology (H&E stain) in Tsc2 iKO mice and control mice (n = 5-6 per group). c) Wright-Giemsa staining of bone marrow leukocytes from Tsc2 iKO and control mice. d) Confocal microscopy and e) electron microscopy of hemophagocytes from the bone marrow of Tsc2 iKO mice. f) Quantification of bone marrow hemophagocytes in Tsc2 iKO mice treated with rapamycin or vehicle control (3 slides analyzed for each mouse; n = 3 per group). Analyses were performed 3 weeks after poly I:C treatment to induce Tsc2 deletion. g) Wright-Giemsa staining of human monocytes with targeted disruption of Tsc2 by CRISPR/Cas9 cultured with M-CSF for 14 days. Disclosures: z. huang, None; x. You, None; L. Chen, None; Y. Du, None; K. Brodeur, None; Q. Wang, None; D. Sykes, Clear Creek Bio; M. Chang, None; J. Charles, None; P. Nigrovic, Bristol-Myers Squibb(BMS), Exo Therapeutics, Miach Orthopedics, Pfizer, Novartis, UpToDate, Cerecor, Brickell Bio; P. Lee, None.

