Back
Poster Session A
Session: (0123–0149) Miscellaneous Rheumatic and Inflammatory Diseases Poster I
0143: National Multicenter Study of 80 Patients with Refractory Uveitis Due to Immune-Mediated Inflammatory Diseases Treated with Certolizumab Pegol
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JM
Jose Luis Martin-Varillas, MD
Hospital Laredo
Laredo, Spain
Abstract Poster Presenter(s)
José Luis Martín-Varillas1, Lara Sánchez-Bilbao2, Vanesa Calvo Río3, Alf Adán4, Inés Hernanz Rodríguez4, Emma Beltrán Catalán5, Sonia Castro Oreiro6, Patricia Fanlo7, Alvaro Garcia8, Ignacio Torre9, Miguel Cordero Coma10, Juan Ramón de Dios11, A. Garcia-Aparicio12, Marisa Hernandez Garfella13, Amalia Sánchez Andrade14, A. García-valle15, Olga Maiz16, Juan Roberto Miguelez Sanchez12, Sergio Rodriguez Montero17, Ana Urruticoechea18, Raul Veroz19, Arantxa Conesa20, Cristina Fernández-Carballido21, Vega Jovani Casano22, Olga Martinez23, Patricia Moya Alvarado24, Susana Romero Yuste25, Paula Rubio Muñoz26, eva Peña Sainz-Pardo27, Marta Garijo Bufort28, José Luis Hernández2 and Ricardo Blanco29, 1Hospital de Laredo, Laredo, Cantabria, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Valdecilla Hospital, Santander, Spain, 4H. Clinic, Barcelona, Spain, 5Hospital del Mar, Barcelona, Spain, 6H. Universitario Joan XXIII, Barcelona, Spain, 7Complejo Hospitalario de Navarra, Navarra, Spain, 8Hospital Tajo, Madrid, Spain, 9Hospital de Basurto, Basurto, Spain, 10Hospital de León, León, Spain, 11Hospital de Araba, Araba, Spain, 12Hospital Universitario de Toledo, Toledo, Spain, 13Hospital Universitario General de Valencia, València, Spain, 14Hospital Universitario Lucus Augusti, Lugo, Spain, 15Completo Asistencial Palencia, Palencia, Spain, 16Hospital Universitario de Donostia, San Sebastián, Spain, 17Virgen de Valme University Hospital, Sevilla, Spain, 18Hospital Can Misses, Ibiza, Spain, 19Hospital de Mérida, Mérida, Spain, 20Hospital Universitario de Castellón, València, Spain, 21Agencia Valenciana de Salud, Alicante, Spain, 22National Health system, Alicante, Spain, 23Hospital Clínico Universitario de Salamanca, Zamora, Spain, 24Hospital Parc Tauli, Barcelona, Spain, 25Complexo Hospitalario Universitario, Pontevedra, Spain, 26H. Esperit Sant, Barcelona, Spain, 27Hospital Universitario 12 de Octubre, Madrid, Spain, 28Hospital Sagunto, València, Spain, 29Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Adalimumab remains the only biologic approved by the EMA and FDA for the treatment of noninfectious uveitis. There are few reports on the effectiveness of other anti-TNF drugs such as certolizumab pegol (CZP). Our aims were to determine the efficacy and safety of CZP in refractory uveitis secondary to Immune-mediated Inflammatory Diseases (IMIDs).
Methods: national multicenter study of 80 patients with uveitis due to IMID refractory to glucocorticoids and conventional immunosuppressants treated with CZP. Efficacy was assessed with the following ocular parameters: best corrected visual acuity (BCVA), anterior chamber cells, vitritis, macular thickness and presence of retinal vasculitis. The efficacy of CZP was compared between the baseline visit, 1st week, 1st and 6th month, and 1st year. Statistical analysis was performed with IBM SPSS Statistics v.23.
Results: we studied 80 patients/111 affected eyes (33 men/47 women) with a mean age of 41.6±11.7 years. The IMIDs included were: spondyloarthritis (n=43), Behçet's disease (10), psoriatic arthritis (8), Crohn's disease (4), sarcoidosis (2), JIA (1), reactive arthritis (1), rheumatoid arthritis (1), relapsing polychondritis (1), TINU (1), pars planitis (1), Birdshot (1) and idiopathic uveitis (6). Anterior was the most frequent uveitis pattern (n=61).
In 20 patients, besides the presence of refractory uveitis, desire of pregnancy was the reason for CZP initiation.
Prior to CZP, patients had received: methotrexate (n=38), sulfasalazine (28), azathioprine (14), cyclosporine (10), leflunomide (3), mycophenolate mofetil (4), and cyclophosphamide (1). Previous biologic therapy was administered in 52 patients (63%), with a median [IQR] of 2 [1-3] drugs per patient. The most used biologic was adalimumab (n=48), followed by infliximab (32), golimumab (15), tocilizumab (5), etanercept (7), rituximab (1), anakinra (1) and secukinumab (1). CZP was administered as monotherapy in 39 patients.
After 24 [12-36] months of follow-up, all parameters analyzed showed a rapid and maintained improvement (TABLE). A decrease in the mean number of uveitis flares was observed before and after CZP, (2.6±2.3 vs. 0.6±0.4, p< 0.001). CZP was discontinued in 16 patients due to: ocular remission (n=3), insufficient ocular response (4) and incomplete response of extraocular manifestations (9). No serious adverse effects were found.
Conclusion: CZP seems to be effective and safe in the control of uveitis associated to different IMIDs.
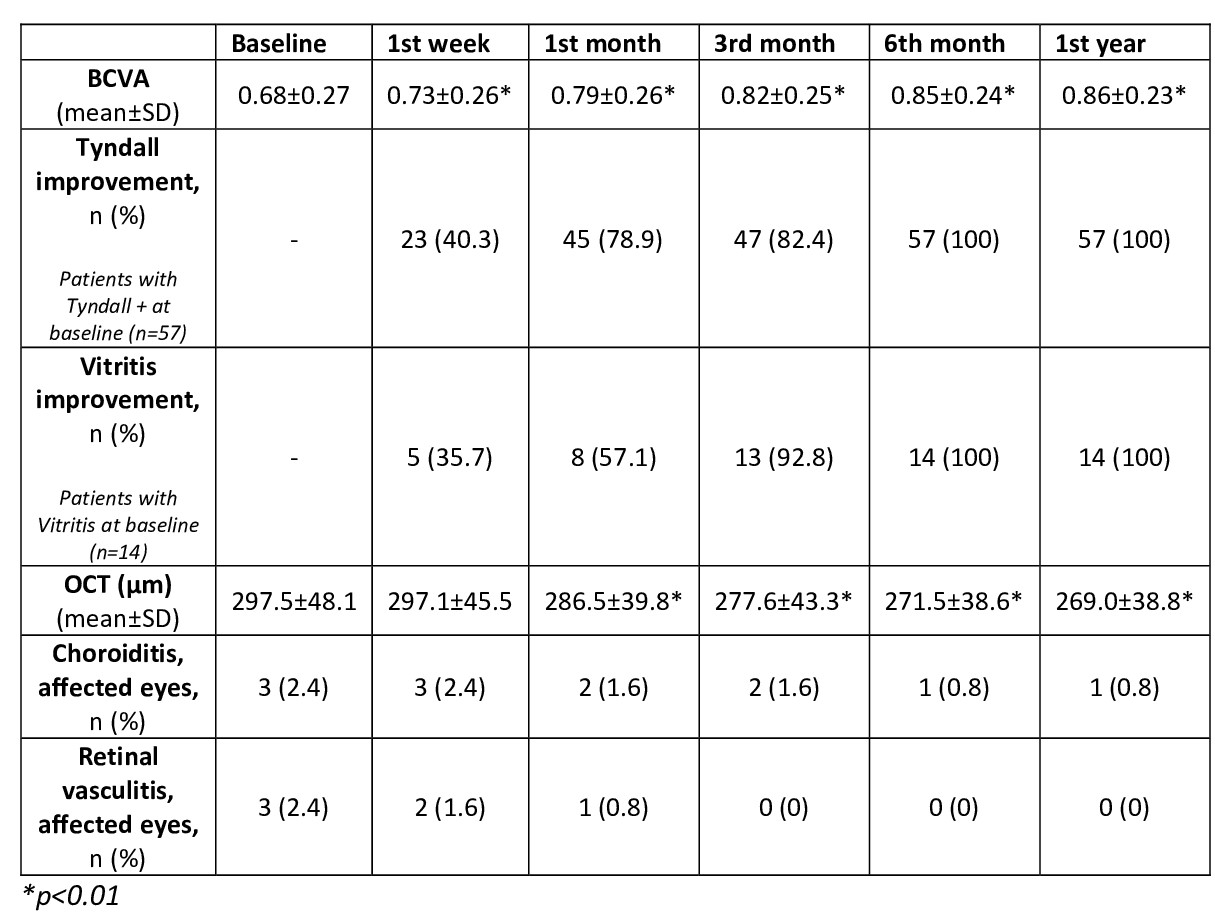 Main ocular parameters analyzed in 80 patients with uveitis due to IMID and treated with CZP.
Main ocular parameters analyzed in 80 patients with uveitis due to IMID and treated with CZP.
Disclosures: J. Martín-Varillas, AbbVie/Abbott, Pfizer, Janssen, UCB, Celgene; L. Sánchez-Bilbao, Eli Lilly; V. Calvo Río, AbbVie/Abbott, Eli Lilly, Merck/MSD, UCB; A. Adán, None; I. Hernanz Rodríguez, None; E. Beltrán Catalán, None; S. Castro Oreiro, None; P. Fanlo, None; A. Garcia, None; I. Torre, None; M. Cordero Coma, None; J. de Dios, None; A. Garcia-Aparicio, None; M. Hernandez Garfella, None; A. Sánchez Andrade, None; A. García-valle, None; O. Maiz, None; J. Miguelez Sanchez, None; S. Rodriguez Montero, None; A. Urruticoechea, None; R. Veroz, None; A. Conesa, None; C. Fernández-Carballido, None; V. Jovani Casano, None; O. Martinez, None; P. Moya Alvarado, None; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; P. Rubio Muñoz, None; e. Peña Sainz-Pardo, None; M. Garijo Bufort, None; J. Hernández, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Adalimumab remains the only biologic approved by the EMA and FDA for the treatment of noninfectious uveitis. There are few reports on the effectiveness of other anti-TNF drugs such as certolizumab pegol (CZP). Our aims were to determine the efficacy and safety of CZP in refractory uveitis secondary to Immune-mediated Inflammatory Diseases (IMIDs).
Methods: national multicenter study of 80 patients with uveitis due to IMID refractory to glucocorticoids and conventional immunosuppressants treated with CZP. Efficacy was assessed with the following ocular parameters: best corrected visual acuity (BCVA), anterior chamber cells, vitritis, macular thickness and presence of retinal vasculitis. The efficacy of CZP was compared between the baseline visit, 1st week, 1st and 6th month, and 1st year. Statistical analysis was performed with IBM SPSS Statistics v.23.
Results: we studied 80 patients/111 affected eyes (33 men/47 women) with a mean age of 41.6±11.7 years. The IMIDs included were: spondyloarthritis (n=43), Behçet's disease (10), psoriatic arthritis (8), Crohn's disease (4), sarcoidosis (2), JIA (1), reactive arthritis (1), rheumatoid arthritis (1), relapsing polychondritis (1), TINU (1), pars planitis (1), Birdshot (1) and idiopathic uveitis (6). Anterior was the most frequent uveitis pattern (n=61).
In 20 patients, besides the presence of refractory uveitis, desire of pregnancy was the reason for CZP initiation.
Prior to CZP, patients had received: methotrexate (n=38), sulfasalazine (28), azathioprine (14), cyclosporine (10), leflunomide (3), mycophenolate mofetil (4), and cyclophosphamide (1). Previous biologic therapy was administered in 52 patients (63%), with a median [IQR] of 2 [1-3] drugs per patient. The most used biologic was adalimumab (n=48), followed by infliximab (32), golimumab (15), tocilizumab (5), etanercept (7), rituximab (1), anakinra (1) and secukinumab (1). CZP was administered as monotherapy in 39 patients.
After 24 [12-36] months of follow-up, all parameters analyzed showed a rapid and maintained improvement (TABLE). A decrease in the mean number of uveitis flares was observed before and after CZP, (2.6±2.3 vs. 0.6±0.4, p< 0.001). CZP was discontinued in 16 patients due to: ocular remission (n=3), insufficient ocular response (4) and incomplete response of extraocular manifestations (9). No serious adverse effects were found.
Conclusion: CZP seems to be effective and safe in the control of uveitis associated to different IMIDs.
 Main ocular parameters analyzed in 80 patients with uveitis due to IMID and treated with CZP.
Main ocular parameters analyzed in 80 patients with uveitis due to IMID and treated with CZP.Disclosures: J. Martín-Varillas, AbbVie/Abbott, Pfizer, Janssen, UCB, Celgene; L. Sánchez-Bilbao, Eli Lilly; V. Calvo Río, AbbVie/Abbott, Eli Lilly, Merck/MSD, UCB; A. Adán, None; I. Hernanz Rodríguez, None; E. Beltrán Catalán, None; S. Castro Oreiro, None; P. Fanlo, None; A. Garcia, None; I. Torre, None; M. Cordero Coma, None; J. de Dios, None; A. Garcia-Aparicio, None; M. Hernandez Garfella, None; A. Sánchez Andrade, None; A. García-valle, None; O. Maiz, None; J. Miguelez Sanchez, None; S. Rodriguez Montero, None; A. Urruticoechea, None; R. Veroz, None; A. Conesa, None; C. Fernández-Carballido, None; V. Jovani Casano, None; O. Martinez, None; P. Moya Alvarado, None; S. Romero Yuste, Pfizer, Lilly, AbbVie, Biogen, Sanofi; P. Rubio Muñoz, None; e. Peña Sainz-Pardo, None; M. Garijo Bufort, None; J. Hernández, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

