Back
Poster Session A
Immunobiology
Session: (0006–0016) B Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
0014: The Expanded CD21 Low B Cell Subpopulation in Ankylosing Spondylitis Consists Mainly of Antigen-Inexperienced Cells
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RW
Rick Wilbrink, MD
University Medical Center Groningen
Groningen, Netherlands
Abstract Poster Presenter(s)
Rick Wilbrink1, Linda van der Weele2, Anneke Spoorenberg1, Niek De Vries2, Frans Kroese1 and Gwenny Verstappen1, 1University Medical Center Groningen, Groningen, Netherlands, 2University Medical Center Amsterdam, Amsterdam, Netherlands
Background/Purpose: The role of B cells in the pathogenesis of ankylosing spondylitis (AS) remains relatively understudied. Nevertheless, available evidence shows presence of B cells at sites of inflammation, higher frequencies of circulating plasmablasts, presence of autoantibodies and promising results of B cell depleting therapy with rituximab. We observed previously that a subpopulation of CD27- B cells, characterized by low expression of the complement receptor CD21 (CD21low), is increased in patients with AS and patients with primary Sjogren's syndrome (pSS), a typical B cell mediated systemic autoimmune disease. These CD21lowCD38lowCD27- B cells display a pro-inflammatory phenotype, suggestive for a pathogenic role. To gain insight into the origin of these CD21lowCD38lowCD27- B cells we analysed the mutation status of the immunoglobulin heavy chain variable (IGHV) gene regions in the total B cell compartment, CD21low B cell subpopulations and plasmablasts in AS patients in comparison to, pSS patients and healthy donors (HD). In addition, we investigated clonal relations between CD21low B cell subpopulations and plasmablasts in AS.
Methods: RNA was isolated from sorted total B cells (CD19+), two CD21low B cell populations (CD21lowCD38lowCD27- and CD21lowCD38lowCD27+) and plasmablasts (CD19+CD38++CD27+) from peripheral mononuclear cells of 10 AS patients fulfilling the mNY criteria (mean age 46 ± 9 years, 60% male, mean ASDAS 2.3 ± 0.8, all HLA-B27+), 10 sex- and age-matched HDs (mean age 48 ± 21 years, 60% male) and 10 age-matched pSS patients (mean age 46 ± 9 years, 20% male). Repertoire analysis of the B cell receptor heavy chain was performed using high throughput sequencing on the Illumina MiSeq Platform. Sequences were analyzed for degree of somatic hypermutation, usage of immunoglobulin heavy chain variable (IGHV) and joining (IGHJ) regions and clonal relationships.
Results: IGHV sequences with identical heavy chain CDR3 regions were grouped together and were defined as a clone. Analysis of the IGHV mutation status in CD21low B cells revealed that the majority of clones (71% SD ± 14.1) in the CD21lowCD38lowCD27- subset from patients with AS consisted of clones with a low mutation rate (0-2 mutations in the IGHV region). On the contrary, most of the clones in the CD21lowCD38lowCD27+ (49% SD ± 12.5) and plasmablasts (59% SD ± 12.7) subsets were clones with a higher mutation load ( >10 mutations in the IGHV region) (Figure 1). A similar mutational pattern was seen in pSS patients and HDs. By performing a CDR3 amino acid network analysis on the top 100 most expanded clones per subset in AS patients we found that the CD21lowCD38lowCD27- subset shared more clones with the CD21lowCD38lowCD27+ subset (median 3.0 (0.8-7.0 IQR) compared to plasmablasts (median 0.0 (0-1.3 IQR) (p< 0.05) (Figure 2).
Conclusion: This study shows that the expanded circulating CD21lowCD38lowCD27- B cells in AS consists mainly of antigen-inexperienced cells. The presence of shared clones with their CD27+ counterpart suggest that the two subsets are closely related. Hence, CD21lowCD38lowCD27- B cells may differentiate towards CD21lowCD38lowCD27+ B cells. The relative roles of these two B cell subsets in AS pathogenesis remains to be established.
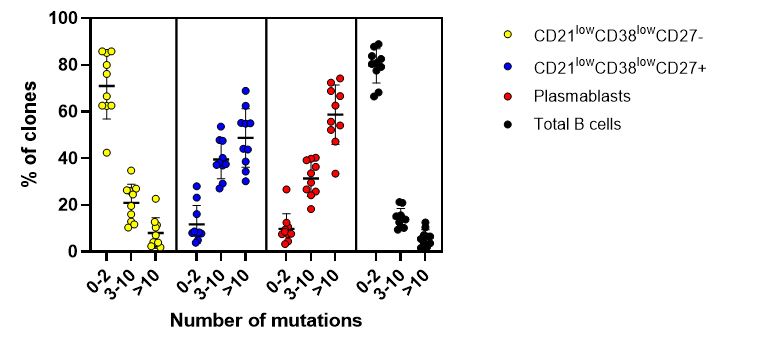 Figure 1. IGHV mutation status per sorted cell subset in ten patients with ankylosing spondylitis. The data are expressed as mean ± standard error of the mean.
Figure 1. IGHV mutation status per sorted cell subset in ten patients with ankylosing spondylitis. The data are expressed as mean ± standard error of the mean.
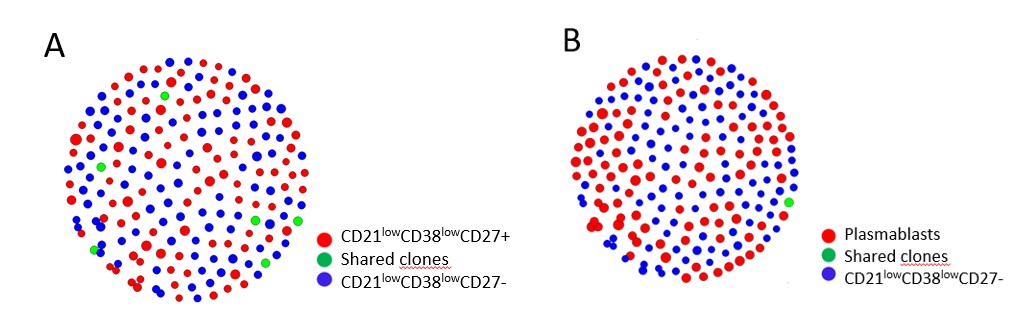 Figure 2. Example of CDR3 amino acid network analysis for 1 patient with ankylosing spondylitis. (A) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and CD21lowCD38lowCD27+ subsets. (B) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and plasmablasts subsets.
Figure 2. Example of CDR3 amino acid network analysis for 1 patient with ankylosing spondylitis. (A) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and CD21lowCD38lowCD27+ subsets. (B) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and plasmablasts subsets.
Disclosures: R. Wilbrink, None; L. van der Weele, None; A. Spoorenberg, Abbvie, Pfizer, Novartis, UCB, Lilly; N. De Vries, None; F. Kroese, None; G. Verstappen, None.
Background/Purpose: The role of B cells in the pathogenesis of ankylosing spondylitis (AS) remains relatively understudied. Nevertheless, available evidence shows presence of B cells at sites of inflammation, higher frequencies of circulating plasmablasts, presence of autoantibodies and promising results of B cell depleting therapy with rituximab. We observed previously that a subpopulation of CD27- B cells, characterized by low expression of the complement receptor CD21 (CD21low), is increased in patients with AS and patients with primary Sjogren's syndrome (pSS), a typical B cell mediated systemic autoimmune disease. These CD21lowCD38lowCD27- B cells display a pro-inflammatory phenotype, suggestive for a pathogenic role. To gain insight into the origin of these CD21lowCD38lowCD27- B cells we analysed the mutation status of the immunoglobulin heavy chain variable (IGHV) gene regions in the total B cell compartment, CD21low B cell subpopulations and plasmablasts in AS patients in comparison to, pSS patients and healthy donors (HD). In addition, we investigated clonal relations between CD21low B cell subpopulations and plasmablasts in AS.
Methods: RNA was isolated from sorted total B cells (CD19+), two CD21low B cell populations (CD21lowCD38lowCD27- and CD21lowCD38lowCD27+) and plasmablasts (CD19+CD38++CD27+) from peripheral mononuclear cells of 10 AS patients fulfilling the mNY criteria (mean age 46 ± 9 years, 60% male, mean ASDAS 2.3 ± 0.8, all HLA-B27+), 10 sex- and age-matched HDs (mean age 48 ± 21 years, 60% male) and 10 age-matched pSS patients (mean age 46 ± 9 years, 20% male). Repertoire analysis of the B cell receptor heavy chain was performed using high throughput sequencing on the Illumina MiSeq Platform. Sequences were analyzed for degree of somatic hypermutation, usage of immunoglobulin heavy chain variable (IGHV) and joining (IGHJ) regions and clonal relationships.
Results: IGHV sequences with identical heavy chain CDR3 regions were grouped together and were defined as a clone. Analysis of the IGHV mutation status in CD21low B cells revealed that the majority of clones (71% SD ± 14.1) in the CD21lowCD38lowCD27- subset from patients with AS consisted of clones with a low mutation rate (0-2 mutations in the IGHV region). On the contrary, most of the clones in the CD21lowCD38lowCD27+ (49% SD ± 12.5) and plasmablasts (59% SD ± 12.7) subsets were clones with a higher mutation load ( >10 mutations in the IGHV region) (Figure 1). A similar mutational pattern was seen in pSS patients and HDs. By performing a CDR3 amino acid network analysis on the top 100 most expanded clones per subset in AS patients we found that the CD21lowCD38lowCD27- subset shared more clones with the CD21lowCD38lowCD27+ subset (median 3.0 (0.8-7.0 IQR) compared to plasmablasts (median 0.0 (0-1.3 IQR) (p< 0.05) (Figure 2).
Conclusion: This study shows that the expanded circulating CD21lowCD38lowCD27- B cells in AS consists mainly of antigen-inexperienced cells. The presence of shared clones with their CD27+ counterpart suggest that the two subsets are closely related. Hence, CD21lowCD38lowCD27- B cells may differentiate towards CD21lowCD38lowCD27+ B cells. The relative roles of these two B cell subsets in AS pathogenesis remains to be established.
 Figure 1. IGHV mutation status per sorted cell subset in ten patients with ankylosing spondylitis. The data are expressed as mean ± standard error of the mean.
Figure 1. IGHV mutation status per sorted cell subset in ten patients with ankylosing spondylitis. The data are expressed as mean ± standard error of the mean. Figure 2. Example of CDR3 amino acid network analysis for 1 patient with ankylosing spondylitis. (A) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and CD21lowCD38lowCD27+ subsets. (B) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and plasmablasts subsets.
Figure 2. Example of CDR3 amino acid network analysis for 1 patient with ankylosing spondylitis. (A) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and CD21lowCD38lowCD27+ subsets. (B) Clonal relationship between the top 100 most expanded clones in the CD21lowCD38lowCD27- and plasmablasts subsets.Disclosures: R. Wilbrink, None; L. van der Weele, None; A. Spoorenberg, Abbvie, Pfizer, Novartis, UCB, Lilly; N. De Vries, None; F. Kroese, None; G. Verstappen, None.

