Back
Poster Session A
Epidemiology, health policy and outcomes
Session: (0187–0213) Patient Outcomes, Preferences, and Attitudes Poster I
0189: Characteristics Associated with a Positive PEST Screening Among Patients with Psoriasis Without a Diagnosis of Psoriatic Arthritis: Results from the Global Psoriasis and Beyond Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Alexis Ogdie, MD
Rheumatologist, Associate Professor of Medicine
University of Pennsylvania
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Alexis Ogdie1, April Armstrong2, Barbra Bohannan3, Sicily Mburu4, Laura Coates5, Elena Kornyeyeva6, Susan Frade6, Silvia Fernandez Barrio7 and Matthias Augustin8, 1Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 2Keck School of Medicine of USC, Dermatology, Los Angeles, CA, 3Psoriasisförbundet, Stockholm, Sweden, 4IFPA, Stockholm, Sweden, 5Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom, 6Novartis Pharma AG, Basel, Switzerland, 7Asociación Para El Enfermo De Psoriasis, Buenos Aires, Argentina, 8University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Background/Purpose: Psoriatic arthritis (PsA) is a common clinical feature in patients (pts) with psoriasis (PsO); up to one third of PsO pts will develop PsA in their lifetime.1 Despite this, the global Psoriasis and Beyond study, conducted by Novartis in partnership with IFPA, recently reported that 71% of PsO pts were unaware of the relationship between their skin disease and PsA. Here we describe the characteristics associated with a positive PEST score among PsO pts without a diagnosis of PsA from the Psoriasis and Beyond study.
Methods: Psoriasis and Beyond was a cross-sectional, quantitative online survey conducted in adults with a self-reported, physician-given diagnosis of moderate to severe PsO (body surface area [BSA] >5 to < 10% affecting sensitive and/or prominent body parts, or BSA ≥10%) at its worst, with/without concomitant PsA regardless of treatment. Pts without a confirmed diagnosis of PsA were screened using the Psoriasis Epidemiology Screening Tool (PEST); positive answers in ≥3 of 5 questions were considered as having a higher probability of undiagnosed PsA (ie, PEST-positive [PEST+] pts).
Results: A total of 4978 responses from 20 countries were analyzed; 3490 pts had a diagnosis of PsO only, while 1488 pts also had a diagnosis of PsA. Of the PsO only pts, 38% (n=1340) screened PEST+ (mean age, 41.3 years). Among pts with diagnosed PsA being PEST+, no difference regarding PsA severity between genders were reported. Women began to experience PsA symptoms at an average age of 32 years (29 years in men); however, more women reported morning stiffness (women vs men: 48% vs 33%).
Of PEST+ pts without PsA diagnosis, PsO severity was categorized as moderate in 26% and severe in 13% of pts. Compared with PEST-negative (PEST−) pts, a higher proportion of PEST+ pts reported dry skin that may crack and bleed (57% vs 44%), soreness around psoriatic patches (36% vs 20%), and thick and pitted nails (16% vs 12%). Sensitive areas, including the palms and soles, were the most affected body parts reported by PEST+ pts (Figure 1). Regardless of the absence of PsA diagnosis, PEST+ (vs PEST−) pts reported having notably swollen joints (89% vs 27%), swollen finger(s) and/or toe(s) (80% vs 15%) and heel pain (72% vs 22%). The knees and hand/finger joints were the predominant joints causing discomfort for PEST+ pts (Figure 2). The comorbidity burden was higher in PEST+ pts with anxiety, depression, and obesity being the most commonly reported (Figure 3). PsO impact on quality of life was also substantial in PEST+ pts, with 38% and 26% of pts reporting a very large and extremely large effect on the patient's life, compared with 22% and 5% of PEST− pts, respectively.
Conclusion: The analysis identified a large sub-population of PsO pts who screened PEST+, indicating a higher probability of having undiagnosed PsA and highlighting the need for active screening for PsA by the PsO-treating physicians to minimize non-reversible joint damage. Further, severe PsO and a high comorbidity burden may be associated with higher PEST scores. These associations should be further investigated in future studies.
Reference 1. Gottlieb, A., Merola J.F. J Dermatolog Treat. 2020;31:662–79.
.jpg) Figure 1. PEST response in self-reported sensitive areas affected by plaque PsO
Figure 1. PEST response in self-reported sensitive areas affected by plaque PsO
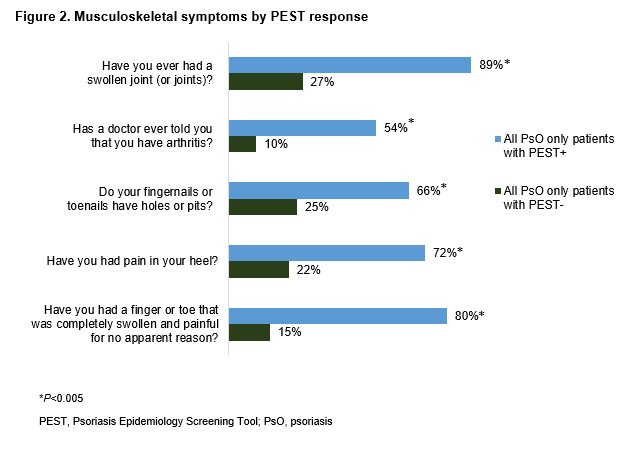 Figure 2. Musculoskeletal symptoms by PEST response
Figure 2. Musculoskeletal symptoms by PEST response
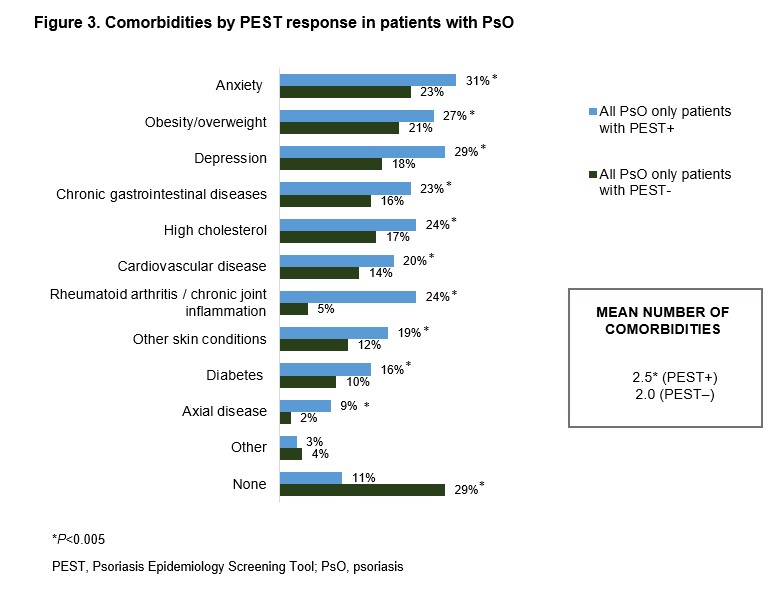 Figure 3. Comorbidities by PEST response in patients with PsO
Figure 3. Comorbidities by PEST response in patients with PsO
Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; A. Armstrong, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb(BMS), EPI, Incyte, Leo Pharma, UCB Pharma, Novartis, Ortho Dermatologics, Sun Pharma, Sanofi, Regeneron, Pfizer, Almirall, Arcutis, Aslan, Janssen, Eli Lilly, Nimbus, Dermavant, Dermira; B. Bohannan, None; S. Mburu, None; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); E. Kornyeyeva, Novartis; S. Frade, Novartis; S. Fernandez Barrio, None; M. Augustin, AbbVie/Abbott, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKlein(GSK), Janssen-Cilag, Leo Pharma, Medac, Merck/MSD, Novartis, Pfizer, UCB, Bayer, Beiersdorf, Bristol-Myers Squibb(BMS), Dermira, Galderma, Genzyme, Hexal, Incyte, Menlo, Mylan B.V, Regeneron, Sandoz, Sanofi, Stallergenes, Trevi.
Background/Purpose: Psoriatic arthritis (PsA) is a common clinical feature in patients (pts) with psoriasis (PsO); up to one third of PsO pts will develop PsA in their lifetime.1 Despite this, the global Psoriasis and Beyond study, conducted by Novartis in partnership with IFPA, recently reported that 71% of PsO pts were unaware of the relationship between their skin disease and PsA. Here we describe the characteristics associated with a positive PEST score among PsO pts without a diagnosis of PsA from the Psoriasis and Beyond study.
Methods: Psoriasis and Beyond was a cross-sectional, quantitative online survey conducted in adults with a self-reported, physician-given diagnosis of moderate to severe PsO (body surface area [BSA] >5 to < 10% affecting sensitive and/or prominent body parts, or BSA ≥10%) at its worst, with/without concomitant PsA regardless of treatment. Pts without a confirmed diagnosis of PsA were screened using the Psoriasis Epidemiology Screening Tool (PEST); positive answers in ≥3 of 5 questions were considered as having a higher probability of undiagnosed PsA (ie, PEST-positive [PEST+] pts).
Results: A total of 4978 responses from 20 countries were analyzed; 3490 pts had a diagnosis of PsO only, while 1488 pts also had a diagnosis of PsA. Of the PsO only pts, 38% (n=1340) screened PEST+ (mean age, 41.3 years). Among pts with diagnosed PsA being PEST+, no difference regarding PsA severity between genders were reported. Women began to experience PsA symptoms at an average age of 32 years (29 years in men); however, more women reported morning stiffness (women vs men: 48% vs 33%).
Of PEST+ pts without PsA diagnosis, PsO severity was categorized as moderate in 26% and severe in 13% of pts. Compared with PEST-negative (PEST−) pts, a higher proportion of PEST+ pts reported dry skin that may crack and bleed (57% vs 44%), soreness around psoriatic patches (36% vs 20%), and thick and pitted nails (16% vs 12%). Sensitive areas, including the palms and soles, were the most affected body parts reported by PEST+ pts (Figure 1). Regardless of the absence of PsA diagnosis, PEST+ (vs PEST−) pts reported having notably swollen joints (89% vs 27%), swollen finger(s) and/or toe(s) (80% vs 15%) and heel pain (72% vs 22%). The knees and hand/finger joints were the predominant joints causing discomfort for PEST+ pts (Figure 2). The comorbidity burden was higher in PEST+ pts with anxiety, depression, and obesity being the most commonly reported (Figure 3). PsO impact on quality of life was also substantial in PEST+ pts, with 38% and 26% of pts reporting a very large and extremely large effect on the patient's life, compared with 22% and 5% of PEST− pts, respectively.
Conclusion: The analysis identified a large sub-population of PsO pts who screened PEST+, indicating a higher probability of having undiagnosed PsA and highlighting the need for active screening for PsA by the PsO-treating physicians to minimize non-reversible joint damage. Further, severe PsO and a high comorbidity burden may be associated with higher PEST scores. These associations should be further investigated in future studies.
Reference 1. Gottlieb, A., Merola J.F. J Dermatolog Treat. 2020;31:662–79.
.jpg) Figure 1. PEST response in self-reported sensitive areas affected by plaque PsO
Figure 1. PEST response in self-reported sensitive areas affected by plaque PsO Figure 2. Musculoskeletal symptoms by PEST response
Figure 2. Musculoskeletal symptoms by PEST response  Figure 3. Comorbidities by PEST response in patients with PsO
Figure 3. Comorbidities by PEST response in patients with PsO Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; A. Armstrong, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb(BMS), EPI, Incyte, Leo Pharma, UCB Pharma, Novartis, Ortho Dermatologics, Sun Pharma, Sanofi, Regeneron, Pfizer, Almirall, Arcutis, Aslan, Janssen, Eli Lilly, Nimbus, Dermavant, Dermira; B. Bohannan, None; S. Mburu, None; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); E. Kornyeyeva, Novartis; S. Frade, Novartis; S. Fernandez Barrio, None; M. Augustin, AbbVie/Abbott, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKlein(GSK), Janssen-Cilag, Leo Pharma, Medac, Merck/MSD, Novartis, Pfizer, UCB, Bayer, Beiersdorf, Bristol-Myers Squibb(BMS), Dermira, Galderma, Genzyme, Hexal, Incyte, Menlo, Mylan B.V, Regeneron, Sandoz, Sanofi, Stallergenes, Trevi.

