Back
Poster Session A
Patient engagement/self-management
Session: (0181–0186) Patient Education/Community Programs Poster
0182: Newly Diagnosed with Inflammatory Arthritis. Development of a Complex Self-management Intervention
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BE
Bente Esbensen, RN, PhD, MSN
Rigshospitalet
Glostrup, Denmark
Abstract Poster Presenter(s)
Luise Lindgren1, Tanja Thomsen2, Mette Aadahl3, Sara Kristensen4, Bente Esbensen5, Annette de Thurah6 and Merete L Hetland5, 1Copenhagen Center for Arthritis Research, Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark, 2Rigshospitalet, Glostrup, Center for Rheumatology and Spine Diseases. COPECARE, Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, Glostrup, Denmark, 3Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, Denmark.Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, Frederiksberg, Denmark, 4Patient research partner, Frederiksberg, Denmark, 5Rigshospitalet, Glostrup, Denmark, 6Department of Rheumatology, Aarhus University Hospital, Denmark. Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Background/Purpose: Patients newly diagnosed with inflammatory arthritis (IA) request regular consultations and support from health professionals (HPRs) in rheumatology to manage physiological, emotional, and social challenges. Evidence suggests that providing a tailored tailormade multi-component self-management program may benefit disease management (1). However, there is a lack of evidence in interventions with multiple components targeting patients newly diagnosed with IA.
Therefore, the purpose of this project was to develop a complex, evidence- and theory-based intervention in collaboration with patients and HPRs for better self-management in newly diagnosed patients suitable for a rheumatology outpatient clinic.
Methods: This study followed the Medical Research Council (MRC) Framework (2,3) for the development of complex interventions. It consists of 4 stages (Figure 1.).
1) Identifying the evidence base
We conducted 2 literature reviews, from which, we described a preliminary nurse-led intervention.
2) Identifying theory
Social Cognitive Theory (4), was chosen as the underlying theory along with Acceptance and Commitment Theory (5) to support our communicative strategy.
3) Modelling process and outcomes
The preliminary intervention was discussed and further developed in 7 workshops to ensure that the intervention was in accordance with patients' needs and feasible in clinical practice. Three patients and 38 HPRs attended. Discussions were digitally recorded and analyzed using Thematic Analysis (6).
Results: 4) Description of all components and outcomes of the intervention
Our intervention, ready for testing in a feasibility study, is a 9-month nurse-led intervention and consists of four individual and two group sessions. A physiotherapist and an occupational therapist will also attend the group sessions along with the nurse (Figure 2). All sessions target IA-specific self-management.
A comprehensive intervention manual was developed. Our patient research partner and experts in rheumatology and self-management commented on the content to secure content validity. Subsequently, we conducted cognitive interviews with the HPRs to determine the face validity of the manual. In addition, we completed a two-day competence program to train HPRs in delivering the intervention.
The selected patient-reported outcomes are Physical activity levels, Health assessment, Fatigue, Quality of life, Anxiety and depression, Illness intrusiveness, Illness perception, and Self-efficacy, supplemented with objective measures for disease activity.
Conclusion: NISMA - a nurse-led complex self-management intervention embedded in a multidisciplinary team has been developed and described based on MRC's framework for the development of complex interventions. The intervention is currently being tested in a feasibility study.
References
1. Nikiphorou et al., Annals of the Rheumatic Diseases. 1. Oct 2021.
2. Skivington et al., BMJ. 30. sep. 2021.
3. Craig et al., nt J Nurs Stud. May 2013.
4. Bandura A. SOCIAL COGNITIVE THEORY.
5. Newman et al., Lancet. Oct 2004.
6. Braun et al., Springer; 2019.
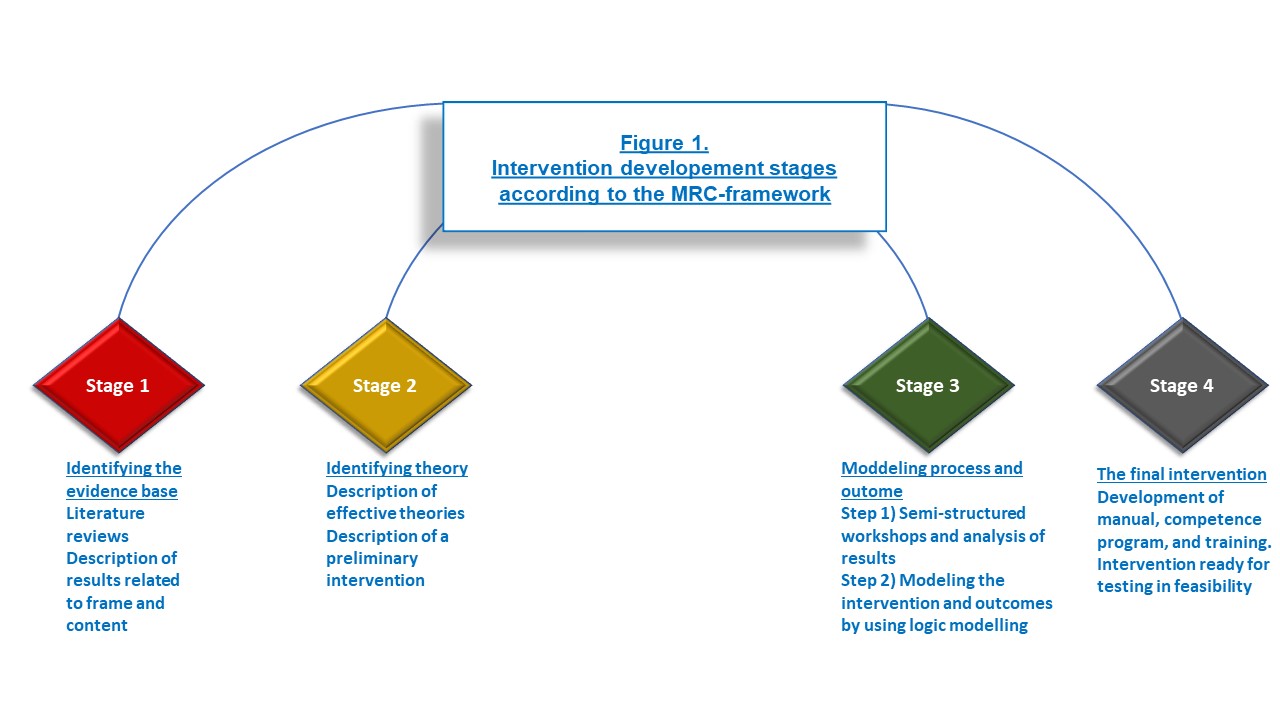 Figure 1. Development stages.
Figure 1. Development stages.
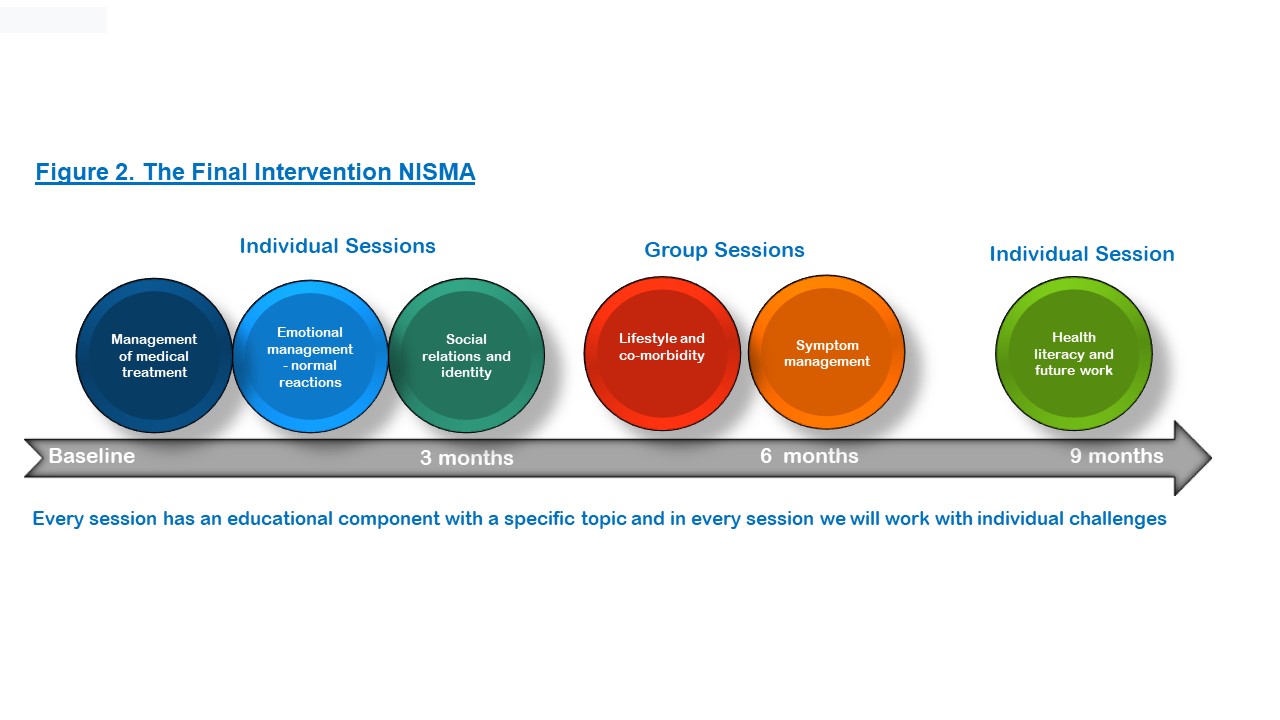 Figure 2. The intervention.
Figure 2. The intervention.
Disclosures: L. Lindgren, None; T. Thomsen, None; M. Aadahl, None; S. Kristensen, None; B. Esbensen, None; A. de Thurah, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac.
Background/Purpose: Patients newly diagnosed with inflammatory arthritis (IA) request regular consultations and support from health professionals (HPRs) in rheumatology to manage physiological, emotional, and social challenges. Evidence suggests that providing a tailored tailormade multi-component self-management program may benefit disease management (1). However, there is a lack of evidence in interventions with multiple components targeting patients newly diagnosed with IA.
Therefore, the purpose of this project was to develop a complex, evidence- and theory-based intervention in collaboration with patients and HPRs for better self-management in newly diagnosed patients suitable for a rheumatology outpatient clinic.
Methods: This study followed the Medical Research Council (MRC) Framework (2,3) for the development of complex interventions. It consists of 4 stages (Figure 1.).
1) Identifying the evidence base
We conducted 2 literature reviews, from which, we described a preliminary nurse-led intervention.
2) Identifying theory
Social Cognitive Theory (4), was chosen as the underlying theory along with Acceptance and Commitment Theory (5) to support our communicative strategy.
3) Modelling process and outcomes
The preliminary intervention was discussed and further developed in 7 workshops to ensure that the intervention was in accordance with patients' needs and feasible in clinical practice. Three patients and 38 HPRs attended. Discussions were digitally recorded and analyzed using Thematic Analysis (6).
Results: 4) Description of all components and outcomes of the intervention
Our intervention, ready for testing in a feasibility study, is a 9-month nurse-led intervention and consists of four individual and two group sessions. A physiotherapist and an occupational therapist will also attend the group sessions along with the nurse (Figure 2). All sessions target IA-specific self-management.
A comprehensive intervention manual was developed. Our patient research partner and experts in rheumatology and self-management commented on the content to secure content validity. Subsequently, we conducted cognitive interviews with the HPRs to determine the face validity of the manual. In addition, we completed a two-day competence program to train HPRs in delivering the intervention.
The selected patient-reported outcomes are Physical activity levels, Health assessment, Fatigue, Quality of life, Anxiety and depression, Illness intrusiveness, Illness perception, and Self-efficacy, supplemented with objective measures for disease activity.
Conclusion: NISMA - a nurse-led complex self-management intervention embedded in a multidisciplinary team has been developed and described based on MRC's framework for the development of complex interventions. The intervention is currently being tested in a feasibility study.
References
1. Nikiphorou et al., Annals of the Rheumatic Diseases. 1. Oct 2021.
2. Skivington et al., BMJ. 30. sep. 2021.
3. Craig et al., nt J Nurs Stud. May 2013.
4. Bandura A. SOCIAL COGNITIVE THEORY.
5. Newman et al., Lancet. Oct 2004.
6. Braun et al., Springer; 2019.
 Figure 1. Development stages.
Figure 1. Development stages.  Figure 2. The intervention.
Figure 2. The intervention. Disclosures: L. Lindgren, None; T. Thomsen, None; M. Aadahl, None; S. Kristensen, None; B. Esbensen, None; A. de Thurah, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac.

