Back
Poster Session A
Session: (0123–0149) Miscellaneous Rheumatic and Inflammatory Diseases Poster I
0140: Continued Treatment with Nintedanib in Patients with Progressive Fibrosing Autoimmune Disease-Related Interstitial Lung Diseases (ILDs): Data from INBUILD-ON
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Eric L. Matteson, MD, MPH
Mayo Clinic College of Medicine and Science
Rochester, MN, United States
Abstract Poster Presenter(s)
Eric Matteson1, Danielle Antin-Ozerkis2, Francesco Bonella3, Nazia Chaudhuri4, Vincent Cottin5, Heiko Müller6, Carl Coeck7, Klaus Rohr8 and Wim Wuyts9, 1Mayo Clinic College of Medicine and Science, Rochester, MN, USA, Rochester, MN, 2Section of Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, New Haven, CT, 3Center for Interstitial and Rare Lung Diseases, Pneumology Department, Ruhrlandklinik, University of Duisburg-Essen, Essen, Germany, 4North West Interstitial Lung Disease Unit, Manchester University Foundation Trust, Wythenshawe, Manchester, United Kingdom, 5Coordinating Reference Center for Rare Pulmonary Diseases, Louis Pradel Hospital, University of Lyon, INRAE, Lyon, France, 6Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany, Biberach, Germany, 7Boehringer Ingelheim SComm, Brussels, Brussels, Belgium, 8Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany, 9Unit for Interstitial Lung Diseases, Department of Pulmonary Medicine, University Hospitals Leuven, Leuven, Belgium, Leuven, Belgium
Background/Purpose: In the INBUILD trial in patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis, nintedanib reduced the rate of decline in forced vital capacity (FVC) compared with placebo, with adverse events that were manageable for most patients. INBUILD-ON is an open-label extension study that is collecting data on adverse events and FVC decline in patients treated with nintedanib over the longer term. We analyzed data from the subgroup of patients with progressive pulmonary fibrosis due to autoimmune disease.
Methods: Patients in the INBUILD trial had diffuse fibrosing ILD (reticular abnormality with traction bronchiectasis, with or without honeycombing) of >10% extent on HRCT, FVC ≥45% predicted, DLco ≥30%–< 80% predicted, and met criteria for progression of ILD within the prior 24 months, despite management deemed appropriate in clinical practice. Patients who completed the INBUILD trial on treatment were eligible to enter INBUILD-ON. Patients who received nintedanib in INBUILD and continued nintedanib in INBUILD-ON comprised the "continued nintedanib" group. Patients who received placebo in INBUILD and initiated nintedanib in INBUILD-ON comprised the "initiated nintedanib" group. Dose reductions and treatment interruptions were allowed to manage adverse events. In descriptive analyses, we analyzed adverse events and changes in FVC in INBUILD-ON in patients with autoimmune disease-related ILDs based on a data snapshot taken on 15 October 2021.
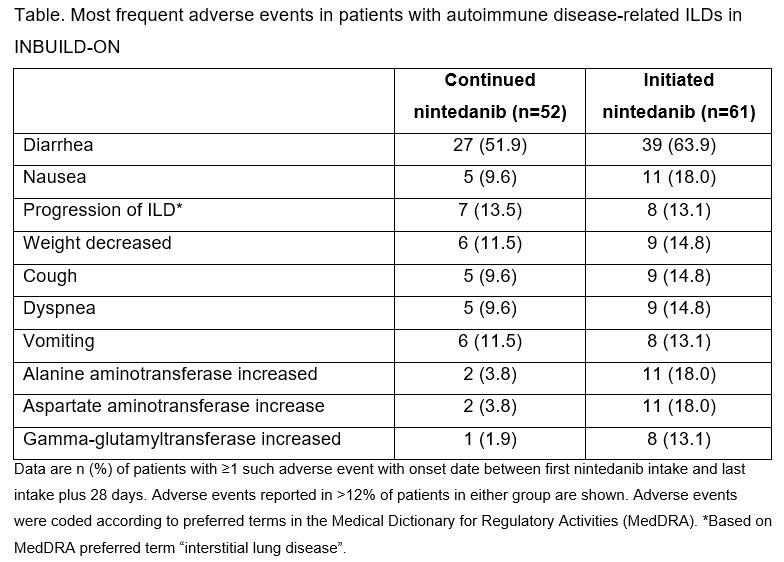
Results: Of the 434 patients treated in INBUILD-ON, 113 had autoimmune disease-related ILDs (52 RA-ILD, 29 SSc-ILD, 13 MCTD-ILD, 19 other autoimmune disease-related ILDs). Of these, 64.6% received ≥1 disease-modifying anti-rheumatic drug or high-dose glucocorticoid ( >20 mg/day prednisone or equivalent) during the trial. The most frequently reported adverse event was diarrhea (Table). Serious adverse events were reported in 26 (50.0%) patients in the continued nintedanib group and 35 (57.4%) patients in the initiated nintedanib group. Among patients who continued and initiated nintedanib, respectively, 7 (13.5%) and 31 (50.8%) had ≥1 dose reduction and 15 (28.8%) and 26 (42.6%) had ≥1 treatment interruption. Adverse events led to discontinuation of nintedanib in 12 (23.1%) patients in the continued nintedanib group and 26 (42.6%) patients in the initiated nintedanib group. Mean (SE) changes in FVC from baseline to week 96 of INBUILD-ON were −61.3 (39.8) mL in the continued nintedanib group and −62.5 (49.4) mL in the initiated nintedanib group (Figure).
Conclusion: The adverse event profile of nintedanib in patients with progressive pulmonary fibrosis related to autoimmune disease in INBUILD-ON was consistent with that reported in the INBUILD trial. Dose adjustments, treatment interruptions and permanent discontinuations of nintedanib were less frequent in patients who continued nintedanib in INBUILD-ON than in those who initiated nintedanib in INBUILD-ON.

Disclosures: E. Matteson, UpToDate, Boehringer Ingelheim, Gilead Sciences, Practice Point Communications, Horizon Therapeutics, American College of Rheumatology; D. Antin-Ozerkis, Boehringer Ingelheim, Fibrogen, Pliant, Galecto, Galapagos, Genentech/Roche, BMS; F. Bonella, Boehringer Ingelheim, Boehringer Ingelheim, Fujirebio, Galapagos NV, Roche, Roche, Bristol Myers Squibb, GlaxoSmithKline, Takeda; N. Chaudhuri, Boehringer Ingelheim, Redx, Liminal BioSciences, Vicore Pharma AB, Bridge Biotherapeutics, tranScrip Ltd; V. Cottin, Boehringer Ingelheim, Roche, Shionogi, RedX, PureTech, Celgene/BMS, AstraZeneca, XSL Behring, Sanofi, United Therapeutics, Pliant, Boehringer Ingelheim, Roche, Galapagos, Celgene/BMS, CSL Behring, Galecto, Fibrogen; H. Müller, Boehringer Ingelheim; C. Coeck, Boehringer Ingelheim; K. Rohr, Boehringer Ingelheim; W. Wuyts, Boehringer Ingelheim, Roche, Galapagos.
Background/Purpose: In the INBUILD trial in patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis, nintedanib reduced the rate of decline in forced vital capacity (FVC) compared with placebo, with adverse events that were manageable for most patients. INBUILD-ON is an open-label extension study that is collecting data on adverse events and FVC decline in patients treated with nintedanib over the longer term. We analyzed data from the subgroup of patients with progressive pulmonary fibrosis due to autoimmune disease.
Methods: Patients in the INBUILD trial had diffuse fibrosing ILD (reticular abnormality with traction bronchiectasis, with or without honeycombing) of >10% extent on HRCT, FVC ≥45% predicted, DLco ≥30%–< 80% predicted, and met criteria for progression of ILD within the prior 24 months, despite management deemed appropriate in clinical practice. Patients who completed the INBUILD trial on treatment were eligible to enter INBUILD-ON. Patients who received nintedanib in INBUILD and continued nintedanib in INBUILD-ON comprised the "continued nintedanib" group. Patients who received placebo in INBUILD and initiated nintedanib in INBUILD-ON comprised the "initiated nintedanib" group. Dose reductions and treatment interruptions were allowed to manage adverse events. In descriptive analyses, we analyzed adverse events and changes in FVC in INBUILD-ON in patients with autoimmune disease-related ILDs based on a data snapshot taken on 15 October 2021.
Results: Of the 434 patients treated in INBUILD-ON, 113 had autoimmune disease-related ILDs (52 RA-ILD, 29 SSc-ILD, 13 MCTD-ILD, 19 other autoimmune disease-related ILDs). Of these, 64.6% received ≥1 disease-modifying anti-rheumatic drug or high-dose glucocorticoid ( >20 mg/day prednisone or equivalent) during the trial. The most frequently reported adverse event was diarrhea (Table). Serious adverse events were reported in 26 (50.0%) patients in the continued nintedanib group and 35 (57.4%) patients in the initiated nintedanib group. Among patients who continued and initiated nintedanib, respectively, 7 (13.5%) and 31 (50.8%) had ≥1 dose reduction and 15 (28.8%) and 26 (42.6%) had ≥1 treatment interruption. Adverse events led to discontinuation of nintedanib in 12 (23.1%) patients in the continued nintedanib group and 26 (42.6%) patients in the initiated nintedanib group. Mean (SE) changes in FVC from baseline to week 96 of INBUILD-ON were −61.3 (39.8) mL in the continued nintedanib group and −62.5 (49.4) mL in the initiated nintedanib group (Figure).
Conclusion: The adverse event profile of nintedanib in patients with progressive pulmonary fibrosis related to autoimmune disease in INBUILD-ON was consistent with that reported in the INBUILD trial. Dose adjustments, treatment interruptions and permanent discontinuations of nintedanib were less frequent in patients who continued nintedanib in INBUILD-ON than in those who initiated nintedanib in INBUILD-ON.


Disclosures: E. Matteson, UpToDate, Boehringer Ingelheim, Gilead Sciences, Practice Point Communications, Horizon Therapeutics, American College of Rheumatology; D. Antin-Ozerkis, Boehringer Ingelheim, Fibrogen, Pliant, Galecto, Galapagos, Genentech/Roche, BMS; F. Bonella, Boehringer Ingelheim, Boehringer Ingelheim, Fujirebio, Galapagos NV, Roche, Roche, Bristol Myers Squibb, GlaxoSmithKline, Takeda; N. Chaudhuri, Boehringer Ingelheim, Redx, Liminal BioSciences, Vicore Pharma AB, Bridge Biotherapeutics, tranScrip Ltd; V. Cottin, Boehringer Ingelheim, Roche, Shionogi, RedX, PureTech, Celgene/BMS, AstraZeneca, XSL Behring, Sanofi, United Therapeutics, Pliant, Boehringer Ingelheim, Roche, Galapagos, Celgene/BMS, CSL Behring, Galecto, Fibrogen; H. Müller, Boehringer Ingelheim; C. Coeck, Boehringer Ingelheim; K. Rohr, Boehringer Ingelheim; W. Wuyts, Boehringer Ingelheim, Roche, Galapagos.

