Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0318: Development and Validation of Algorithms to Identify Cutaneous Lupus Patients Using Diagnostic Codes and Prescription Data
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LG
Lisa Guo, MD
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Lisa Guo1, Jordan Said2, Vinod Nambudiri3 and Joseph Merola3, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Boston, MA, 3Harvard Medical School, Brigham and Women's Hospital, Boston, MA
Background/Purpose: There is a paucity of epidemiological studies of cutaneous lupus erythematosus (CLE), which may be due in part to the lack of well-validated methods to identify CLE cases within large datasets such as claims databases. The purpose of this study was to develop and validate the performance of novel algorithms consisting of diagnostic codes and prescription data to identify individuals with CLE.
Methods: We identified potential study patients by querying the Mass General Brigham hospital network's clinical data repository. We selected patients with at least one International Classification of Diseases (ICD)-10 code for cutaneous lupus, including L93.0 (discoid lupus erythematosus), L93.1 (subacute lupus erythematosus) and L93.2 (other local lupus erythematosus) from January 2016 to April 2021. Of the 1902 unique patients identified, we randomly selected a sample of 300 patients to comprise the testing cohort. We developed 12 validation algorithms to test (Table 1) based on prior studies of comparable inflammatory diseases such as systemic lupus or psoriasis. These algorithms consisted of various permutations of lupus-relevant ICD-10 codes, prescriptions for disease-specific medications (such as antimalarials) and the specialty of the coding provider. We created subsets of patients who met the criteria stipulated by each potential algorithm and determined whether each patient truly had CLE based on manual chart review of the electronic medical record. We defined a "true" case of CLE as a patient with documentation of a CLE diagnosis by a dermatologist or a rheumatologist. Positive predictive values (PPVs) were then developed for each proposed algorithm, with p-values calculated using one-sample proportion tests.
Results: Characteristics of the 300 study patients are listed in Table 2. The most common CLE ICD-10 code was L93.0. PPVs per algorithm ranged from 54.14% to 90.68% (Table 3). Algorithms that included an ICD-10 code for CLE from a dermatologist had the highest PPVs. The greater the number of ICD-10 codes for CLE, the higher the PPV, though marginally so. Including prior antimalarial prescriptions also boosted PPV.
Conclusion: We found that algorithms with at least 1 ICD-10 code for CLE from a dermatologist offered acceptable PPV. In particular, the two algorithms with the highest PPVs were 1) at least 3 CLE codes with one from a dermatologist and 2) prior antimalarial prescription with a CLE code from a dermatologist. Validated algorithms that accurately identify CLE cases will allow for further study of CLE patients using electronic medical records, claims data and large real-world databases.
.jpeg) Table 1. Algorithms for identifying patients with cutaneous lupus erythematosus (CLE)
Table 1. Algorithms for identifying patients with cutaneous lupus erythematosus (CLE)
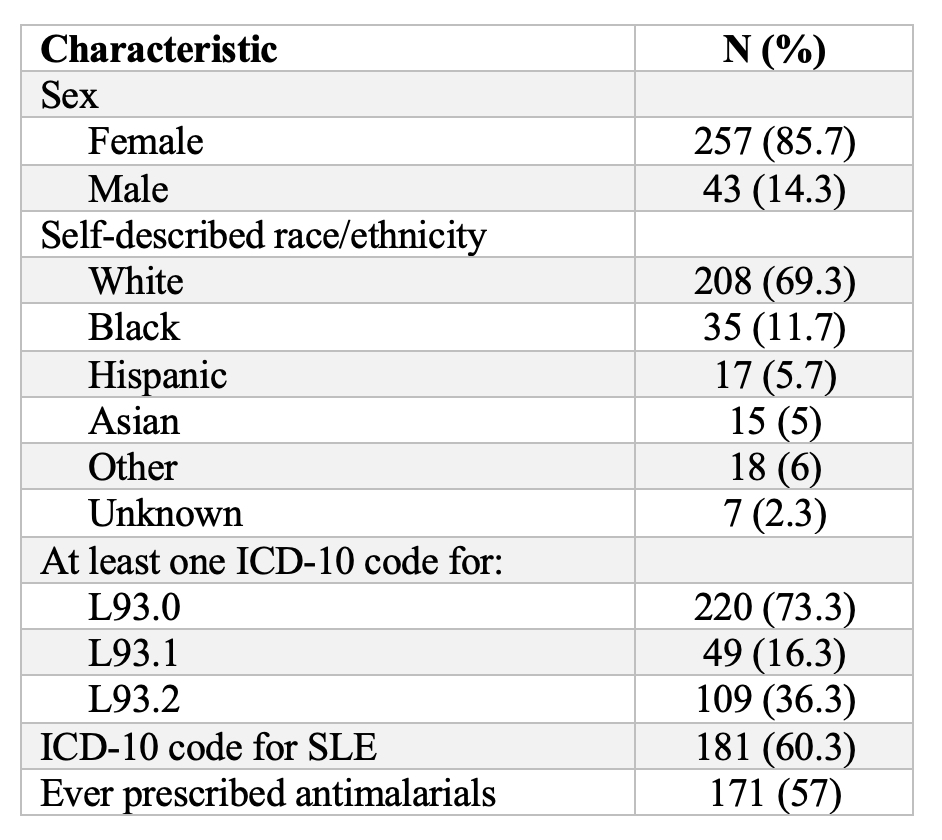 Table 2. Characteristics of patients
Table 2. Characteristics of patients
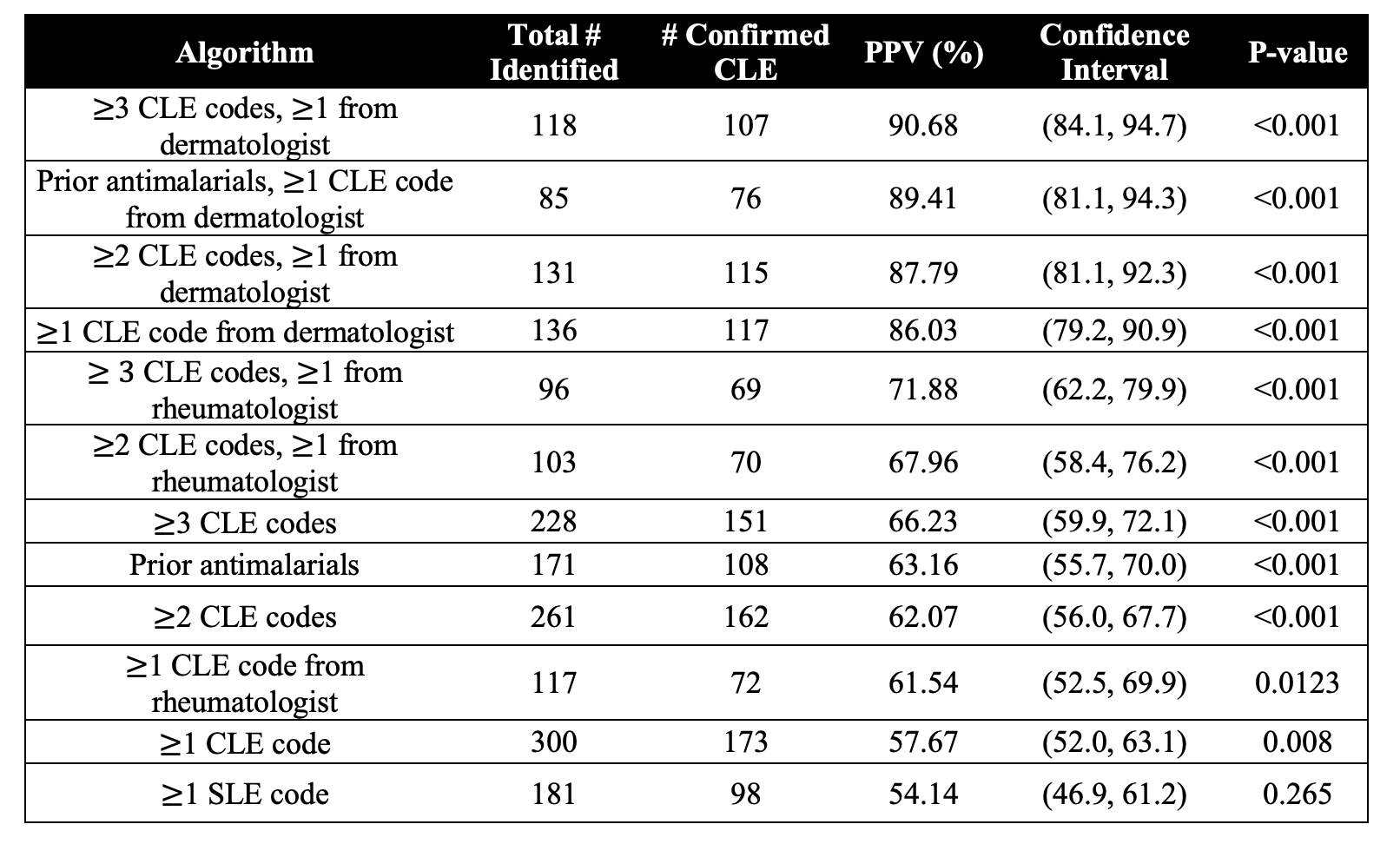 Table 3. Positive predictive values (PPV) of potential algorithms
Table 3. Positive predictive values (PPV) of potential algorithms
Disclosures: L. Guo, None; J. Said, None; V. Nambudiri, Imaging Endpoints, McGraw-Hill; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi.
Background/Purpose: There is a paucity of epidemiological studies of cutaneous lupus erythematosus (CLE), which may be due in part to the lack of well-validated methods to identify CLE cases within large datasets such as claims databases. The purpose of this study was to develop and validate the performance of novel algorithms consisting of diagnostic codes and prescription data to identify individuals with CLE.
Methods: We identified potential study patients by querying the Mass General Brigham hospital network's clinical data repository. We selected patients with at least one International Classification of Diseases (ICD)-10 code for cutaneous lupus, including L93.0 (discoid lupus erythematosus), L93.1 (subacute lupus erythematosus) and L93.2 (other local lupus erythematosus) from January 2016 to April 2021. Of the 1902 unique patients identified, we randomly selected a sample of 300 patients to comprise the testing cohort. We developed 12 validation algorithms to test (Table 1) based on prior studies of comparable inflammatory diseases such as systemic lupus or psoriasis. These algorithms consisted of various permutations of lupus-relevant ICD-10 codes, prescriptions for disease-specific medications (such as antimalarials) and the specialty of the coding provider. We created subsets of patients who met the criteria stipulated by each potential algorithm and determined whether each patient truly had CLE based on manual chart review of the electronic medical record. We defined a "true" case of CLE as a patient with documentation of a CLE diagnosis by a dermatologist or a rheumatologist. Positive predictive values (PPVs) were then developed for each proposed algorithm, with p-values calculated using one-sample proportion tests.
Results: Characteristics of the 300 study patients are listed in Table 2. The most common CLE ICD-10 code was L93.0. PPVs per algorithm ranged from 54.14% to 90.68% (Table 3). Algorithms that included an ICD-10 code for CLE from a dermatologist had the highest PPVs. The greater the number of ICD-10 codes for CLE, the higher the PPV, though marginally so. Including prior antimalarial prescriptions also boosted PPV.
Conclusion: We found that algorithms with at least 1 ICD-10 code for CLE from a dermatologist offered acceptable PPV. In particular, the two algorithms with the highest PPVs were 1) at least 3 CLE codes with one from a dermatologist and 2) prior antimalarial prescription with a CLE code from a dermatologist. Validated algorithms that accurately identify CLE cases will allow for further study of CLE patients using electronic medical records, claims data and large real-world databases.
.jpeg) Table 1. Algorithms for identifying patients with cutaneous lupus erythematosus (CLE)
Table 1. Algorithms for identifying patients with cutaneous lupus erythematosus (CLE) Table 2. Characteristics of patients
Table 2. Characteristics of patients  Table 3. Positive predictive values (PPV) of potential algorithms
Table 3. Positive predictive values (PPV) of potential algorithmsDisclosures: L. Guo, None; J. Said, None; V. Nambudiri, Imaging Endpoints, McGraw-Hill; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi.

