Back
Poster Session A
Vasculitis
Session: (0458–0497) Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
0460: Baseline Glucocorticoid Toxicity in the Treatment of Giant Cell Arteritis: A Post Hoc Analysis of the GiACTA Trial
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Naomi Patel, MD
Massachusetts General Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Naomi Patel1, Xiaoqing Fu2, yuqing zhang3 and John Stone4, 1Massachusetts General Hospital, Sale Creek, TN, 2Massachusetts General Hospital, Boston, MA, 3Massachusetts General Hospital, Quincy, MA, 4Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA
Background/Purpose: Giant cell arteritis (GCA) requires treatment with high-dose, long-term glucocorticoids (GCs). The development of future GC toxicities may be predicted by baseline toxicities. Thus, a baseline GC toxicity score can serve as an important covariate in analyses and play an essential role in risk stratification for patients initiating GCs and potential GC-sparing therapies. However, baseline GC toxicity has not been assessed systematically in rheumatic disease patients thus far.
Methods: We analyzed GCA patients enrolled in the GiACTA (Tocilizumab in Giant Cell Arteritis) trial. The screening period for GiACTA was up to 42 days, during which time patients were treated with GCs as necessary to control their GCA. Baseline GC toxicity scores for different domains were calculated from the medical history, laboratory values, vital signs, and medications. Weights for the items within domains were derived from the Glucocorticoid Toxicity Index (GTI), which includes 9 domains: Body Mass Index, Glucose Tolerance, Blood Pressure, Lipid Metabolism, Bone/Tendon, Glucocorticoid Myopathy, Skin, Neuropsychiatric, and Infection, as well as 3 additional damage domains: Ocular, Gastrointestinal, and Endocrine (Table 1). The overall baseline GC toxicity scores ranged potentially from 0 to 538, with higher scores indicating greater toxicity. We calculated the breakdown of scores within each domain and assessed the contributions of each domain score to the overall score by evaluating the sum total of scores within each domain divided by the sum total of all overall scores. We used a t-test to compare differences in overall baseline GC toxicity scores and Mantel-Haenszel Chi-squared test to evaluate differences in baseline GC toxicity domain scores between those with newly-diagnosed versus relapsing disease at baseline.
Results: A total of 250 patients were included (75% females, mean age 69 years). The mean ± SD baseline GC toxicity score among all patients was 111.3 ± 53.2. The domains that contributed most to the overall scores were the Blood Pressure domain (24.0% of the overall score), followed by Glucose Tolerance (22.6%), and then Neuropsychiatric (15.9%). Baseline GC toxicity scores were higher in patients with relapsing disease compared with those with newly-diagnosed disease (mean of 122.5 vs. 98.9, P< 0.001) (Table 2; Figure). The Body Mass Index and Neuropsychiatric domain scores were significantly higher in those with relapsing disease.
Conclusion: The Blood Pressure and Glucose Tolerance domains contributed most significantly to baseline GC toxicity scores in patients with GCA. Baseline GC toxicity scores were significantly higher in patients with relapsing as opposed to newly-diagnosed disease. Baseline GC toxicity scores may have important implications for risk-stratification in patients starting GC therapy.
.jpg) Table 1. Components of baseline GC toxicity scores and the Glucocorticoid Toxicity Index.
Table 1. Components of baseline GC toxicity scores and the Glucocorticoid Toxicity Index.
.jpg) Table 2. Differences in baseline glucocorticoid toxicity scores between those with newly-diagnosed versus relapsing disease at baseline.
Table 2. Differences in baseline glucocorticoid toxicity scores between those with newly-diagnosed versus relapsing disease at baseline.
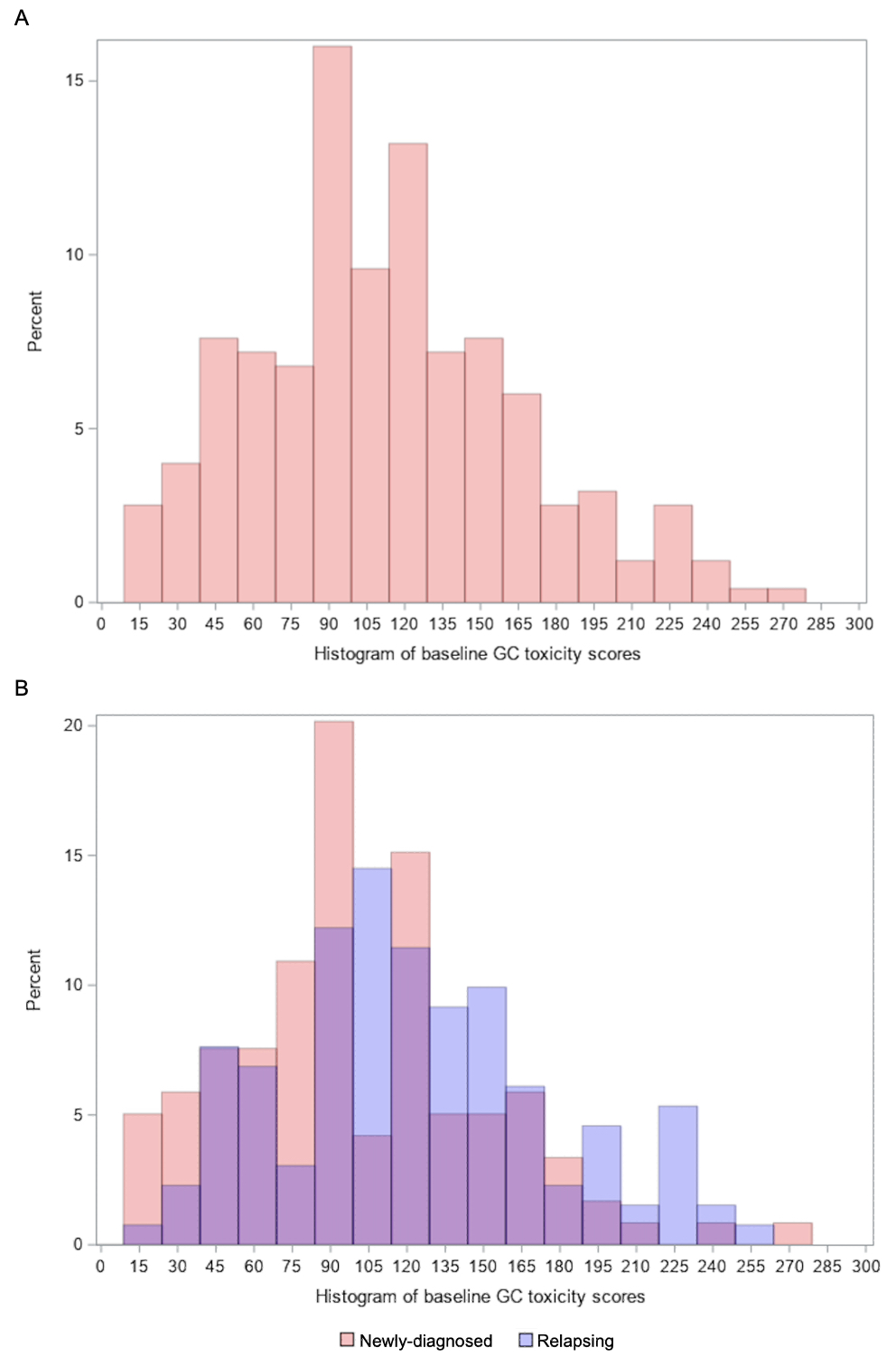 Figure 1. Histogram of baseline glucocorticoid toxicity scores in all patients as well as those with newly-diagnosed versus relapsing disease at baseline.
Figure 1. Histogram of baseline glucocorticoid toxicity scores in all patients as well as those with newly-diagnosed versus relapsing disease at baseline.
A. Distribution of baseline GC toxicity scores in all patients. B. Distribution of baseline GC toxicity scores in patients with newly-diagnosed versus relapsing disease. Purple represents overlap in the percentages of newly-diagnosed and relapsing patients. Red represents excess percentage of newly-diagnosed patients over those with relapsing disease. Blue represents excess percentage of patients with relapsing disease over those with newly-diagnosed disease.
GC, glucocorticoid
Disclosures: N. Patel, FVC Health; X. Fu, None; y. zhang, None; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas.
Background/Purpose: Giant cell arteritis (GCA) requires treatment with high-dose, long-term glucocorticoids (GCs). The development of future GC toxicities may be predicted by baseline toxicities. Thus, a baseline GC toxicity score can serve as an important covariate in analyses and play an essential role in risk stratification for patients initiating GCs and potential GC-sparing therapies. However, baseline GC toxicity has not been assessed systematically in rheumatic disease patients thus far.
Methods: We analyzed GCA patients enrolled in the GiACTA (Tocilizumab in Giant Cell Arteritis) trial. The screening period for GiACTA was up to 42 days, during which time patients were treated with GCs as necessary to control their GCA. Baseline GC toxicity scores for different domains were calculated from the medical history, laboratory values, vital signs, and medications. Weights for the items within domains were derived from the Glucocorticoid Toxicity Index (GTI), which includes 9 domains: Body Mass Index, Glucose Tolerance, Blood Pressure, Lipid Metabolism, Bone/Tendon, Glucocorticoid Myopathy, Skin, Neuropsychiatric, and Infection, as well as 3 additional damage domains: Ocular, Gastrointestinal, and Endocrine (Table 1). The overall baseline GC toxicity scores ranged potentially from 0 to 538, with higher scores indicating greater toxicity. We calculated the breakdown of scores within each domain and assessed the contributions of each domain score to the overall score by evaluating the sum total of scores within each domain divided by the sum total of all overall scores. We used a t-test to compare differences in overall baseline GC toxicity scores and Mantel-Haenszel Chi-squared test to evaluate differences in baseline GC toxicity domain scores between those with newly-diagnosed versus relapsing disease at baseline.
Results: A total of 250 patients were included (75% females, mean age 69 years). The mean ± SD baseline GC toxicity score among all patients was 111.3 ± 53.2. The domains that contributed most to the overall scores were the Blood Pressure domain (24.0% of the overall score), followed by Glucose Tolerance (22.6%), and then Neuropsychiatric (15.9%). Baseline GC toxicity scores were higher in patients with relapsing disease compared with those with newly-diagnosed disease (mean of 122.5 vs. 98.9, P< 0.001) (Table 2; Figure). The Body Mass Index and Neuropsychiatric domain scores were significantly higher in those with relapsing disease.
Conclusion: The Blood Pressure and Glucose Tolerance domains contributed most significantly to baseline GC toxicity scores in patients with GCA. Baseline GC toxicity scores were significantly higher in patients with relapsing as opposed to newly-diagnosed disease. Baseline GC toxicity scores may have important implications for risk-stratification in patients starting GC therapy.
.jpg) Table 1. Components of baseline GC toxicity scores and the Glucocorticoid Toxicity Index.
Table 1. Components of baseline GC toxicity scores and the Glucocorticoid Toxicity Index..jpg) Table 2. Differences in baseline glucocorticoid toxicity scores between those with newly-diagnosed versus relapsing disease at baseline.
Table 2. Differences in baseline glucocorticoid toxicity scores between those with newly-diagnosed versus relapsing disease at baseline. Figure 1. Histogram of baseline glucocorticoid toxicity scores in all patients as well as those with newly-diagnosed versus relapsing disease at baseline.
Figure 1. Histogram of baseline glucocorticoid toxicity scores in all patients as well as those with newly-diagnosed versus relapsing disease at baseline.A. Distribution of baseline GC toxicity scores in all patients. B. Distribution of baseline GC toxicity scores in patients with newly-diagnosed versus relapsing disease. Purple represents overlap in the percentages of newly-diagnosed and relapsing patients. Red represents excess percentage of newly-diagnosed patients over those with relapsing disease. Blue represents excess percentage of patients with relapsing disease over those with newly-diagnosed disease.
GC, glucocorticoid
Disclosures: N. Patel, FVC Health; X. Fu, None; y. zhang, None; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas.

