Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0345: Association Between Cumulated Hydroxychloroquine in Systemic Lupus Erythematosus and Development of Cardiac Conduction Alterations: A Multiple Logistic Regression Analysis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AH
Alba Herrero-Morant, MD
Hospital Universitario Marqués de Valdecilla
Ontinyent, Spain
Abstract Poster Presenter(s)
Alba Herrero-Morant1, Jon Zubiaur-Zamacola2, Adrián Margarida-De Castro2, Raquel Pérez-Barquín2, Miguel Ángel González-Gay3 and Ricardo Blanco4, 1Hospital Universitario Marqués de Valdecilla, Ontinyent, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 4Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Hydroxychloroquine (HCQ) is a widely used drug in Systemic Lupus Erythematosus (SLE). It may cause cardiac alterations which includes short term arrhythmic events (via QT interval prolongation) and medium-late term dose dependent cardiomyopathy. The few research articles published on the medium-late term effects of HCQ in cardiac conduction disorder do not show relevant alterations. The purpose of the study was to assess the effect of HCQ in cardiac conduction in a consecutive SLE population.
Methods: Observational, single University hospital study of all consecutive SLE patients with an electrocardiogram (EKG) at HCQ onset and at least one EKG in follow-up, with a period of at least 3 months on HCQ treatment was performed. We assessed conduction alteration (CA) by EKG, defined as atrio-ventricular block, bundle branch block or QT interval prolongation. The EKGs were gathered from the clinical history and interpreted at the beginning of the treatment and during the 15.2 years (CI95% 13.24-17.16) follow-up period. We defined cumulated HCQ (cHCQ) as the multiple of the mean annual dose of the sample. A Multiple logistic regression model, adjusted by different variables according to statistical significance and clinical relevance, was performed.<
Results: We studied 109 (96 women/13 men) SLE patients with a mean (±SD) age of 61 ±. 2.78 years. A statistically significant association was observed between the cHCQ, and the development of CA [OR 1.1 CI95% 1.02-1.9; p = 0.011] (TABLE & FIGURE). A total of 8 covariates were included. Among them, those that had the greatest influence on the development of the primary event were previous CA [OR 4.15 CI95% 6.39-624.54; p < 0.01]; valvular heart disease [OR 7.15 CI95% 1.31-38.91; p = 0.023] and age [OR 1.07 95% CI 1.0-1.14; p = 0.04].
Conclusion: According to our study, it seems to be an association between the cHCQ and development of CA regardless of other variables evaluated. Wider longitudinal studies are required with a protocolized EKG performance in successive visits to further analyze this association.
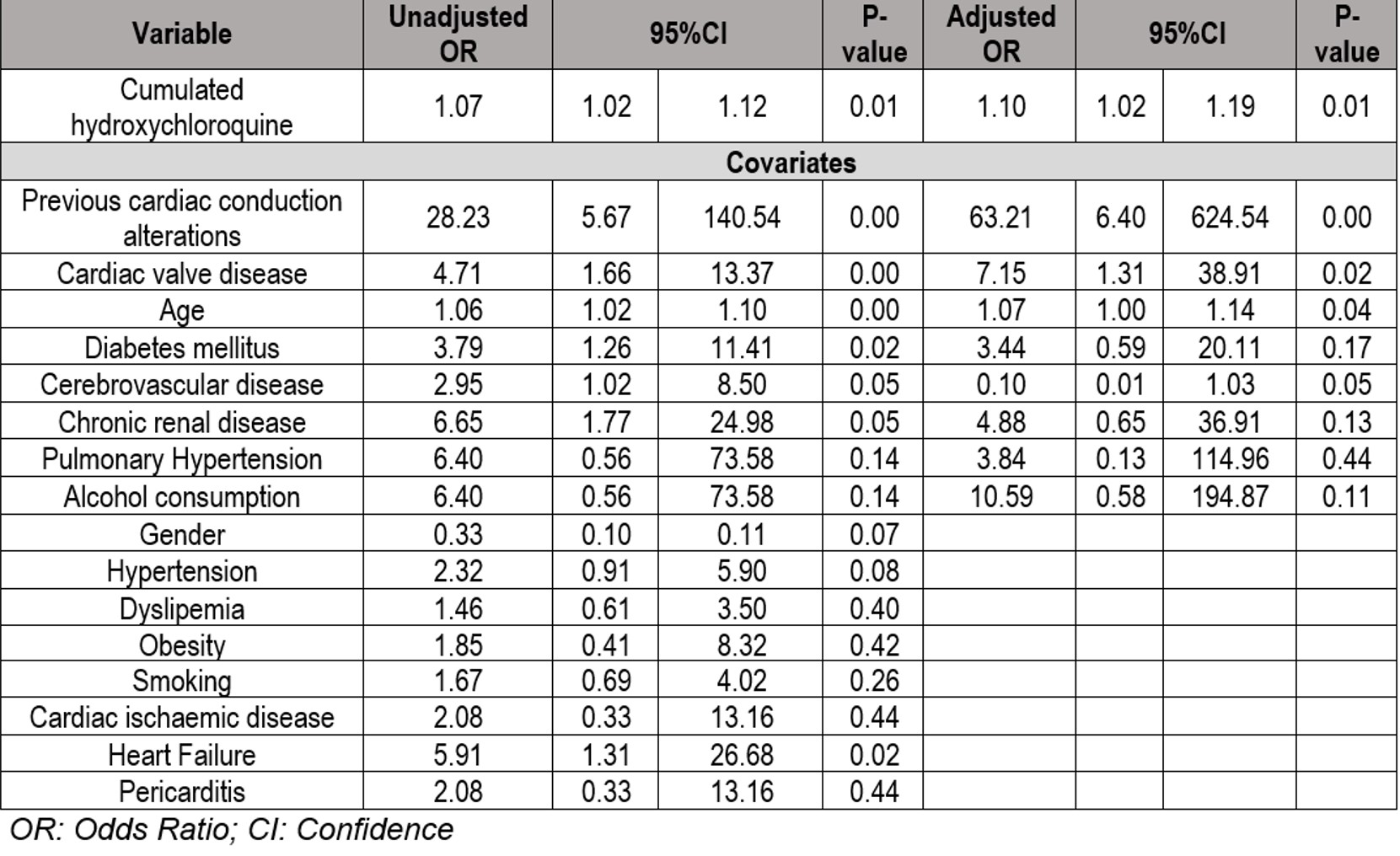 TABLE: Results of univariable and multivariable logistic regressions evaluating the association between cumulated Hydroxychloroquine and the development of cardiac conduction alterations
TABLE: Results of univariable and multivariable logistic regressions evaluating the association between cumulated Hydroxychloroquine and the development of cardiac conduction alterations
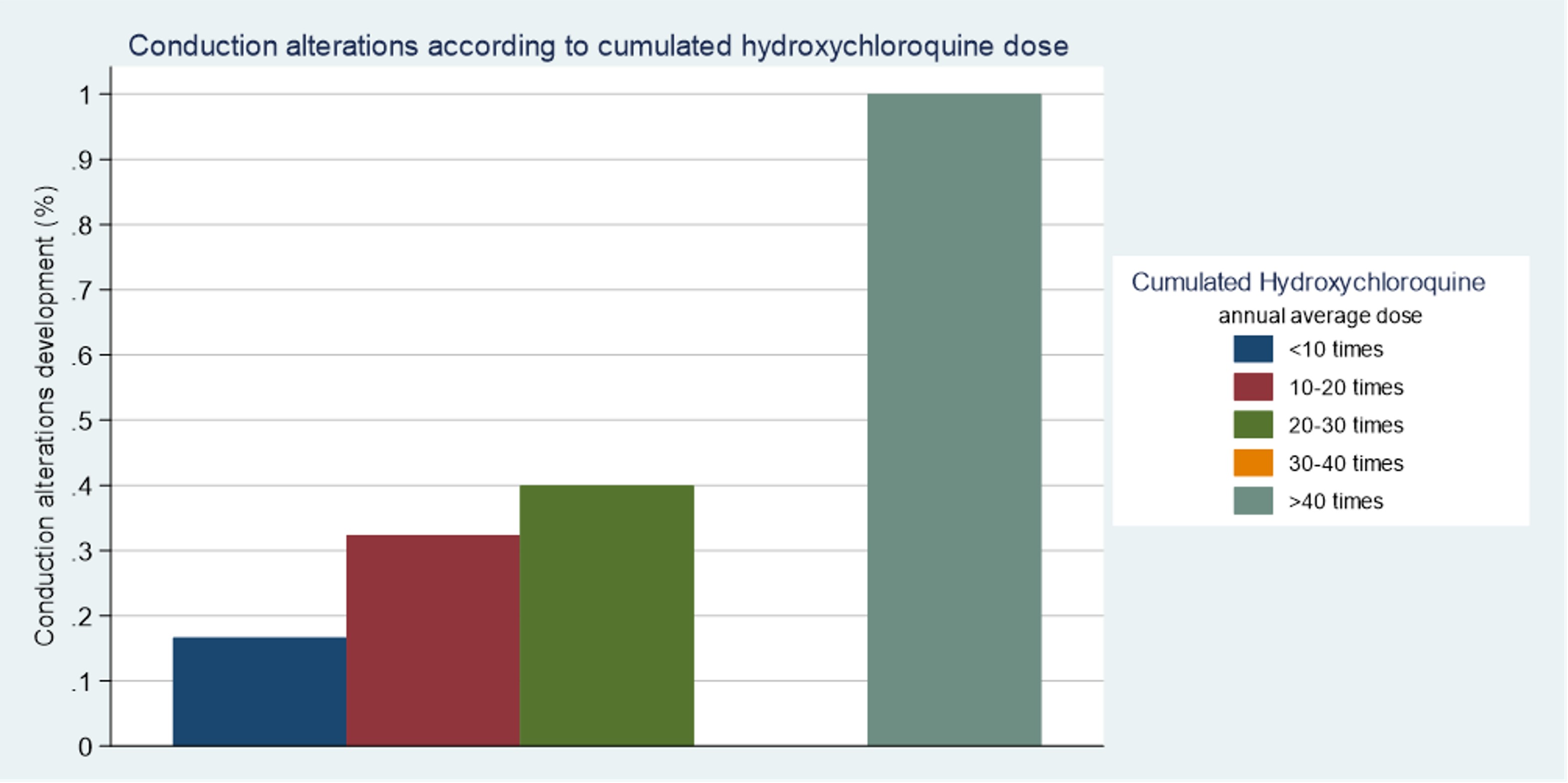 FIGURE: Cardiac conduction alterations development according to cumulated Hydroxychloroquine dose
FIGURE: Cardiac conduction alterations development according to cumulated Hydroxychloroquine dose
Disclosures: A. Herrero-Morant, None; J. Zubiaur-Zamacola, None; A. Margarida-De Castro, None; R. Pérez-Barquín, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.
Background/Purpose: Hydroxychloroquine (HCQ) is a widely used drug in Systemic Lupus Erythematosus (SLE). It may cause cardiac alterations which includes short term arrhythmic events (via QT interval prolongation) and medium-late term dose dependent cardiomyopathy. The few research articles published on the medium-late term effects of HCQ in cardiac conduction disorder do not show relevant alterations. The purpose of the study was to assess the effect of HCQ in cardiac conduction in a consecutive SLE population.
Methods: Observational, single University hospital study of all consecutive SLE patients with an electrocardiogram (EKG) at HCQ onset and at least one EKG in follow-up, with a period of at least 3 months on HCQ treatment was performed. We assessed conduction alteration (CA) by EKG, defined as atrio-ventricular block, bundle branch block or QT interval prolongation. The EKGs were gathered from the clinical history and interpreted at the beginning of the treatment and during the 15.2 years (CI95% 13.24-17.16) follow-up period. We defined cumulated HCQ (cHCQ) as the multiple of the mean annual dose of the sample. A Multiple logistic regression model, adjusted by different variables according to statistical significance and clinical relevance, was performed.<
Results: We studied 109 (96 women/13 men) SLE patients with a mean (±SD) age of 61 ±. 2.78 years. A statistically significant association was observed between the cHCQ, and the development of CA [OR 1.1 CI95% 1.02-1.9; p = 0.011] (TABLE & FIGURE). A total of 8 covariates were included. Among them, those that had the greatest influence on the development of the primary event were previous CA [OR 4.15 CI95% 6.39-624.54; p < 0.01]; valvular heart disease [OR 7.15 CI95% 1.31-38.91; p = 0.023] and age [OR 1.07 95% CI 1.0-1.14; p = 0.04].
Conclusion: According to our study, it seems to be an association between the cHCQ and development of CA regardless of other variables evaluated. Wider longitudinal studies are required with a protocolized EKG performance in successive visits to further analyze this association.
 TABLE: Results of univariable and multivariable logistic regressions evaluating the association between cumulated Hydroxychloroquine and the development of cardiac conduction alterations
TABLE: Results of univariable and multivariable logistic regressions evaluating the association between cumulated Hydroxychloroquine and the development of cardiac conduction alterations FIGURE: Cardiac conduction alterations development according to cumulated Hydroxychloroquine dose
FIGURE: Cardiac conduction alterations development according to cumulated Hydroxychloroquine doseDisclosures: A. Herrero-Morant, None; J. Zubiaur-Zamacola, None; A. Margarida-De Castro, None; R. Pérez-Barquín, None; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi.

