Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0244: Identification of Novel Response Predictors via Cardiovascular Biomarker Proteomic Analysis of Serum Samples from Patients with Early, Seropositive RA Treated with Abatacept or Adalimumab
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
- JL
Jinqi LIU, PhD
Bristol Myers Squibb
Princeton, NJ, United States
Abstract Poster Presenter(s)
Yicong Li1, Chun Wu2, Michael Maldonado2, Peter Schafer2, S. Louis Bridges, Jr.3, William Rigby4, Vivian Bykerk3, Jane Buckner5 and Jinqi Liu2, 1Parexel International, Durham, NC, 2Bristol Myers Squibb, Princeton, NJ, 3Hospital for Special Surgery, New York, NY, 4Dartmouth Hitchcock Medical Center, Lebanon, PA, 5Benaroya Research Institute at Virginia Mason, Seattle, WA
Background/Purpose: Effective RA therapies should not only improve joint signs and symptoms, but also mitigate additional disease-related consequences, such as the increased risk of cardiovascular (CV) disease.1 Although novel DMARD therapies effectively control RA disease activity, the impact on CV risk appears variable.2 Identifying validated predictive response biomarkers (BM) associated with existing therapies would advance clinical decision making and guide development for precision medicine approaches. This exploratory analysis assessed the utility of serum CV BM for predicting clinical response in patients with RA from the Early AMPLE (Abatacept versus adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX) study.3
Methods: Early AMPLE (NCT02557100) was a phase 4 head-to-head comparison of treatment response to either abatacept or adalimumab in patients with early RA with an inadequate response to MTX and dual seropositivity for RF and high ACPA titers, predicted to favor response to abatacept.3 Data for this analysis were generated by the Olink Target 96 multiplex immunoassay platform CV II/III panels (with proximity extension assay), using serum samples collected at baseline (BL) and week 24 for both treatments. Disease activity was monitored using high sensitivity-CRP, tender and swollen joint count, DAS28 (CRP), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index. Relationships between CV BM and disease activity were examined via Spearman's correlations. The prediction model included age, ACPA positivity, BL disease activity, and shared epitope as covariates.
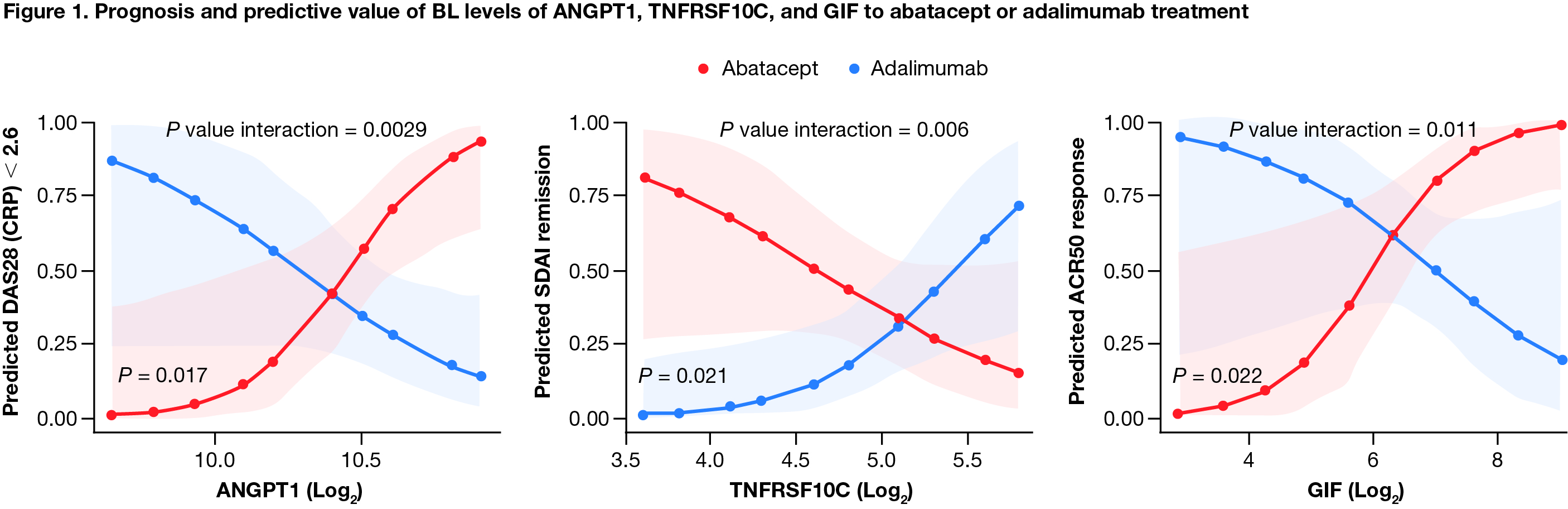
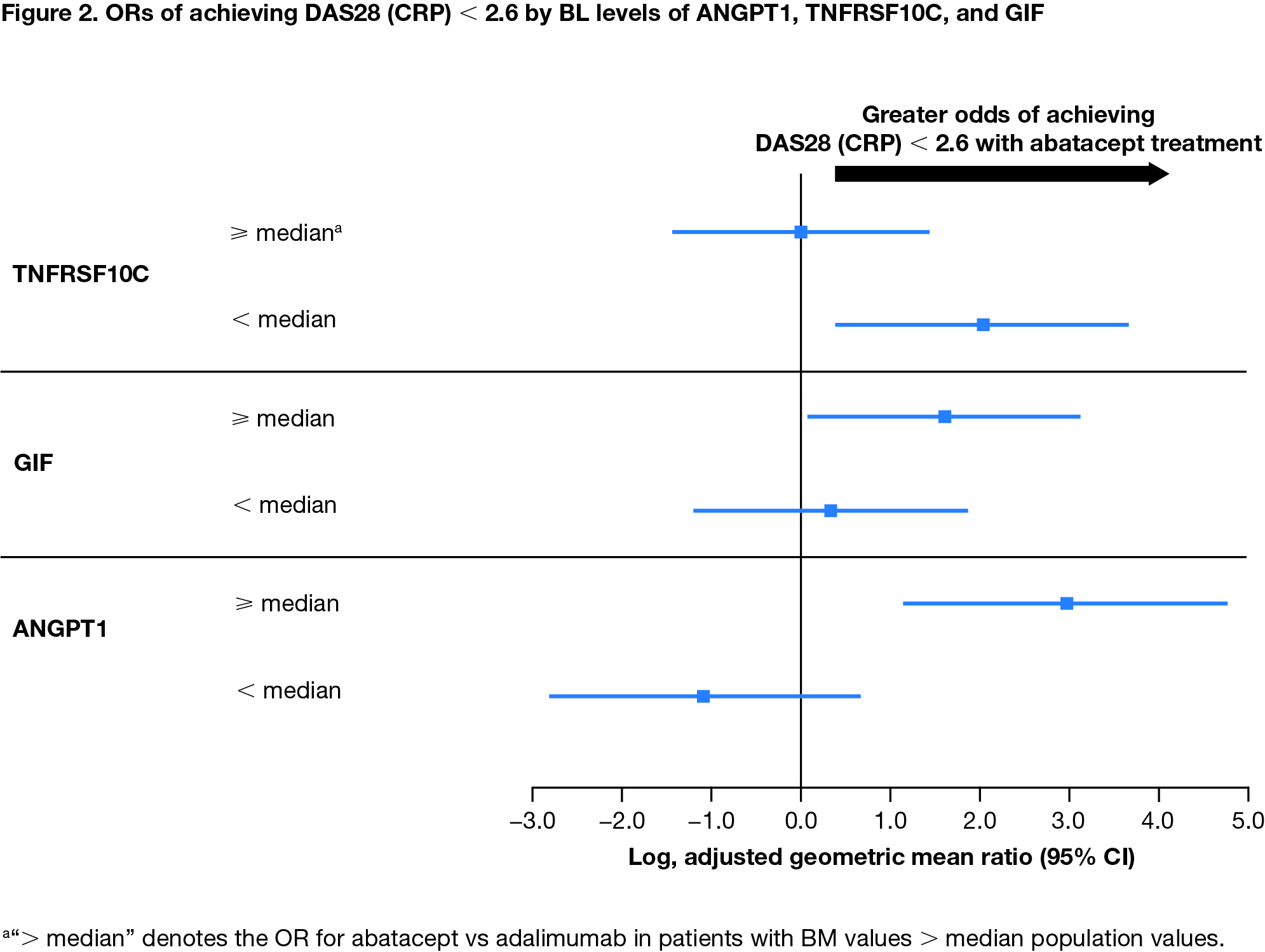
Results: Among all patients, IL-2 receptor α (IL2RA) was the only CV BM (out of 179 analyzed) that positively correlated with all disease activity measures at BL (Spearman's ρ = 0.24–0.58). Change from BL in IL2RA and E-selectin positively correlated with improvement in disease activity at week 24 (all patients; ρ = 0.27-0.39 and 0.31-0.45, respectively). BL levels of 3 CV BM, angiopoietin-1 (ANGPT1), TNF receptor superfamily member 10C (TNFRSF10C), and gastric intrinsic factor (GIF) showed opposite patterns of association with treatment response for abatacept vs adalimumab (Figure 1). Low BL levels of TNFRSF10C and high BL levels of ANGPT1 and GIF indicated a higher likelihood of achieving DAS28 (CRP) < 2.6 in patients treated with abatacept (Figure 2). High levels of ANGPT1 (odds ratio [OR] 2.13 [95% confidence interval (CI) 0.47–3.78]) and GIF (1.93 [0.27–3.60]) and low levels of TNFRSF10C (1.89 [0.14–3.64]) correlated with a higher likelihood of achieving SDAI remission for patients treated with abatacept.
Conclusion: Only 1 CV BM correlated with BL RA disease activity, while 3 CV BM (ANGPT1, TNFRSF10C, and GIF) were identified as potential BM for predicting clinical response by treatment arm. These results uncover novel links between CV BM and RA differential response to abatacept and adalimumab, providing new avenues toward continued investigation of precision medicine approaches.
1England BR, et al. BMJ 2018;361:k1036.
2Santos-Moreno P, et al. RMD Open 2021;7:e001470.
3Rigby W, et al. Arthritis Res Ther 2021;23:245.
Medical writing: Ryan Miller (Caudex), funded by Bristol Myers Squibb.
Disclosures: Y. Li, None; C. Wu, Bristol Myers Squibb; M. Maldonado, Bristol Myers Squibb; P. Schafer, Bristol Myers Squibb; S. Bridges, Jr., Bristol Myers Squibb; W. Rigby, Bristol Myers Squibb, AbbVie; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; J. Liu, Bristol Myers Squibb.
Background/Purpose: Effective RA therapies should not only improve joint signs and symptoms, but also mitigate additional disease-related consequences, such as the increased risk of cardiovascular (CV) disease.1 Although novel DMARD therapies effectively control RA disease activity, the impact on CV risk appears variable.2 Identifying validated predictive response biomarkers (BM) associated with existing therapies would advance clinical decision making and guide development for precision medicine approaches. This exploratory analysis assessed the utility of serum CV BM for predicting clinical response in patients with RA from the Early AMPLE (Abatacept versus adaliMumab comParison in bioLogic-naïvE RA subjects with background MTX) study.3
Methods: Early AMPLE (NCT02557100) was a phase 4 head-to-head comparison of treatment response to either abatacept or adalimumab in patients with early RA with an inadequate response to MTX and dual seropositivity for RF and high ACPA titers, predicted to favor response to abatacept.3 Data for this analysis were generated by the Olink Target 96 multiplex immunoassay platform CV II/III panels (with proximity extension assay), using serum samples collected at baseline (BL) and week 24 for both treatments. Disease activity was monitored using high sensitivity-CRP, tender and swollen joint count, DAS28 (CRP), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index. Relationships between CV BM and disease activity were examined via Spearman's correlations. The prediction model included age, ACPA positivity, BL disease activity, and shared epitope as covariates.
Results: Among all patients, IL-2 receptor α (IL2RA) was the only CV BM (out of 179 analyzed) that positively correlated with all disease activity measures at BL (Spearman's ρ = 0.24–0.58). Change from BL in IL2RA and E-selectin positively correlated with improvement in disease activity at week 24 (all patients; ρ = 0.27-0.39 and 0.31-0.45, respectively). BL levels of 3 CV BM, angiopoietin-1 (ANGPT1), TNF receptor superfamily member 10C (TNFRSF10C), and gastric intrinsic factor (GIF) showed opposite patterns of association with treatment response for abatacept vs adalimumab (Figure 1). Low BL levels of TNFRSF10C and high BL levels of ANGPT1 and GIF indicated a higher likelihood of achieving DAS28 (CRP) < 2.6 in patients treated with abatacept (Figure 2). High levels of ANGPT1 (odds ratio [OR] 2.13 [95% confidence interval (CI) 0.47–3.78]) and GIF (1.93 [0.27–3.60]) and low levels of TNFRSF10C (1.89 [0.14–3.64]) correlated with a higher likelihood of achieving SDAI remission for patients treated with abatacept.
Conclusion: Only 1 CV BM correlated with BL RA disease activity, while 3 CV BM (ANGPT1, TNFRSF10C, and GIF) were identified as potential BM for predicting clinical response by treatment arm. These results uncover novel links between CV BM and RA differential response to abatacept and adalimumab, providing new avenues toward continued investigation of precision medicine approaches.
1England BR, et al. BMJ 2018;361:k1036.
2Santos-Moreno P, et al. RMD Open 2021;7:e001470.
3Rigby W, et al. Arthritis Res Ther 2021;23:245.
Medical writing: Ryan Miller (Caudex), funded by Bristol Myers Squibb.


Disclosures: Y. Li, None; C. Wu, Bristol Myers Squibb; M. Maldonado, Bristol Myers Squibb; P. Schafer, Bristol Myers Squibb; S. Bridges, Jr., Bristol Myers Squibb; W. Rigby, Bristol Myers Squibb, AbbVie; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron; J. Buckner, Amgen, Bristol Myers Squibb, Gentiobio, Hot Spot Therapeutics, Janssen, Pfizer, Novo Nordisk, Allen Institute for Immunology, Type 1 Diabetes TrialNet Study Group, La Jolla Institute, Oklahoma Medical Research Foundation, Bristol Myers Squibb Immunology, Colton Center for Autoimmunity at Penn, Board of Scientific Counsellors; J. Liu, Bristol Myers Squibb.

