Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0245: Influence of Forced Vital Capacity Impairment on Treatment Selection and Outcomes in RA-ILD Patients Initiating a Biologic or Targeted-Synthetic DMARD
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Bryant England, MD, PhD, RhMSUS
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Bryant England1, Michael George2, Yangyuna Yang1, Punyasha Roul3, Jorge Rojas4, Brian Sauer5, Grant Cannon6, Joshua Baker7, Jeffrey Curtis8 and Ted Mikuls9, 1University of Nebraska Medical Center, Omaha, NE, 2University of Pennsylvania, Philadelphia, PA, 3UNMC, Omaha, NE, 4George E. Wahlen Department of Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, 5Salt Lake City VA/University of Utah, Salt Lake City, UT, 6Salt Lake City VA, Salt Lake city, 7University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 8Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 9Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Patients with RA-associated interstitial lung disease (RA-ILD) suffer from substantial morbidity and premature mortality. The optimal use of biologic/tsDMARDs in this population is poorly understood. We evaluated biologic/tsDMARD treatment patterns and outcomes in RA-ILD.
Methods: We identified patients with RA-ILD initiating their first biologic or tsDMARD in the Veterans Health Administration after a diagnosis of ILD between 2006 and 2018. RA-ILD was classified using validated administrative algorithms requiring multiple RA and ILD diagnostic codes (PPV >70%). Pre-treatment initiation FVC values were obtained from pulmonary function test output or extracted from clinical notes using a validated natural language processing program, then categorized as >80%, 60-80%, < 60% predicted, or missing. Patient demographics, health care utilization, comorbidities, and several RA-/ILD-related factors were extracted from EHR and administrative data warehouses. Respiratory-related hospitalizations and vital status were identified during the 3-years after treatment initiation using VA administrative data linked to Medicare and National Death Index data. We evaluated the associations of FVC values with biologic/tsDMARD selection (non-TNFi/JAKi vs. TNFi) and treatment outcomes (respiratory hospitalization, death) in multivariable regression models.
Results: We studied 1,046 RA-ILD patients (mean age 68 years, 92% male), of which 67% initiated a TNFi and 33% a non-TNFi/tsDMARD, including rituximab (n=169), abatacept (n=95), tofacitinib (n=44), and tocilizumab (n=34). Patients with severe FVC impairment (< 60%) more commonly received a non-TNFi/JAKi, although differences were modest and not significant after multivariable adjustment (Table 1). Factors associated with non-TNFi/JAKi selection (referent to TNFi) were female gender, non-White race, more recent calendar years, higher Elixhauser comorbidity scores, more frequent rheumatology and pulmonology visits, and number of prior DMARDs. Baseline use of methotrexate, sulfasalazine, and NSAIDs was associated with TNFi treatment. Within 3-years of treatment initiation, 31% (n=320) had a respiratory-related hospitalization and 24% (n=254) died. RA-ILD patients with a baseline FVC < 60% predicted were at increased risk of respiratory-related hospitalization (HR 1.67 [1.17, 2.36]) and death (HR 1.64 [1.09, 2.45]) compared to those with FVC >80% (Table 2). Additional baseline predictors of respiratory hospitalization and/or death were older age, glucocorticoid use, RF/CCP positivity, elevated ESR/CRP, inhaled corticosteroid use, and baseline home oxygen use. Baseline methotrexate use was associated with a reduced risk of death.
Conclusion: Severe FVC impairment was predictive of respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD but did not strongly influence treatment selection. Despite poor outcomes in patients with severe FVC impairment, providers do not appear to select b/tsDMARDs differently which may reflect the paucity of comparative effectiveness and safety data in RA-ILD.
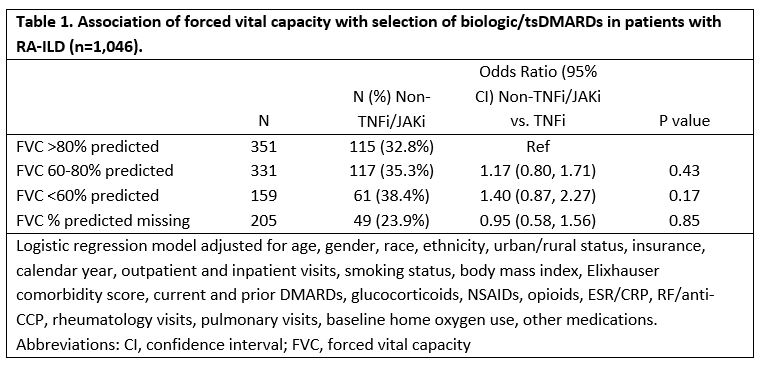 Table 1. Association of forced vital capacity with selection of biologic/tsDMARDs in patients with RA-ILD (n=1,046).
Table 1. Association of forced vital capacity with selection of biologic/tsDMARDs in patients with RA-ILD (n=1,046).
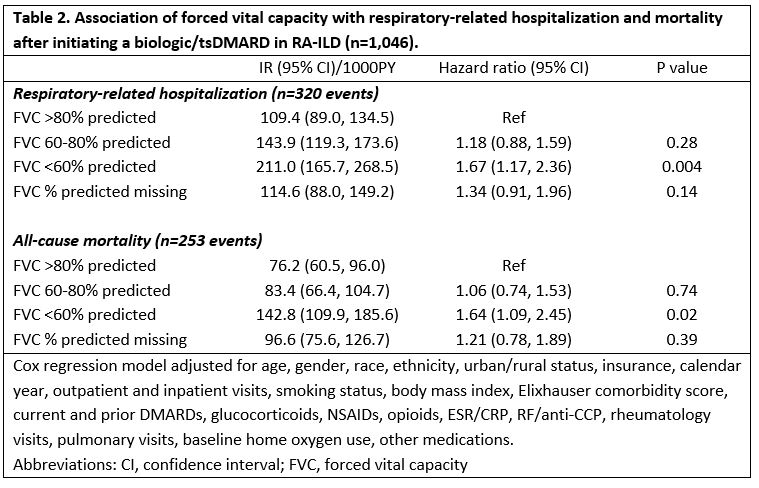 Table 2. Association of forced vital capacity with respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD in RA-ILD (n=1,046).
Table 2. Association of forced vital capacity with respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD in RA-ILD (n=1,046).
Disclosures: B. England, Boehringer-Ingelheim; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; Y. Yang, None; P. Roul, None; J. Rojas, None; B. Sauer, None; G. Cannon, None; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.
Background/Purpose: Patients with RA-associated interstitial lung disease (RA-ILD) suffer from substantial morbidity and premature mortality. The optimal use of biologic/tsDMARDs in this population is poorly understood. We evaluated biologic/tsDMARD treatment patterns and outcomes in RA-ILD.
Methods: We identified patients with RA-ILD initiating their first biologic or tsDMARD in the Veterans Health Administration after a diagnosis of ILD between 2006 and 2018. RA-ILD was classified using validated administrative algorithms requiring multiple RA and ILD diagnostic codes (PPV >70%). Pre-treatment initiation FVC values were obtained from pulmonary function test output or extracted from clinical notes using a validated natural language processing program, then categorized as >80%, 60-80%, < 60% predicted, or missing. Patient demographics, health care utilization, comorbidities, and several RA-/ILD-related factors were extracted from EHR and administrative data warehouses. Respiratory-related hospitalizations and vital status were identified during the 3-years after treatment initiation using VA administrative data linked to Medicare and National Death Index data. We evaluated the associations of FVC values with biologic/tsDMARD selection (non-TNFi/JAKi vs. TNFi) and treatment outcomes (respiratory hospitalization, death) in multivariable regression models.
Results: We studied 1,046 RA-ILD patients (mean age 68 years, 92% male), of which 67% initiated a TNFi and 33% a non-TNFi/tsDMARD, including rituximab (n=169), abatacept (n=95), tofacitinib (n=44), and tocilizumab (n=34). Patients with severe FVC impairment (< 60%) more commonly received a non-TNFi/JAKi, although differences were modest and not significant after multivariable adjustment (Table 1). Factors associated with non-TNFi/JAKi selection (referent to TNFi) were female gender, non-White race, more recent calendar years, higher Elixhauser comorbidity scores, more frequent rheumatology and pulmonology visits, and number of prior DMARDs. Baseline use of methotrexate, sulfasalazine, and NSAIDs was associated with TNFi treatment. Within 3-years of treatment initiation, 31% (n=320) had a respiratory-related hospitalization and 24% (n=254) died. RA-ILD patients with a baseline FVC < 60% predicted were at increased risk of respiratory-related hospitalization (HR 1.67 [1.17, 2.36]) and death (HR 1.64 [1.09, 2.45]) compared to those with FVC >80% (Table 2). Additional baseline predictors of respiratory hospitalization and/or death were older age, glucocorticoid use, RF/CCP positivity, elevated ESR/CRP, inhaled corticosteroid use, and baseline home oxygen use. Baseline methotrexate use was associated with a reduced risk of death.
Conclusion: Severe FVC impairment was predictive of respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD but did not strongly influence treatment selection. Despite poor outcomes in patients with severe FVC impairment, providers do not appear to select b/tsDMARDs differently which may reflect the paucity of comparative effectiveness and safety data in RA-ILD.
 Table 1. Association of forced vital capacity with selection of biologic/tsDMARDs in patients with RA-ILD (n=1,046).
Table 1. Association of forced vital capacity with selection of biologic/tsDMARDs in patients with RA-ILD (n=1,046). Table 2. Association of forced vital capacity with respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD in RA-ILD (n=1,046).
Table 2. Association of forced vital capacity with respiratory-related hospitalization and mortality after initiating a biologic/tsDMARD in RA-ILD (n=1,046).Disclosures: B. England, Boehringer-Ingelheim; M. George, AbbVie, GlaxoSmithKlein(GSK), Chemocentryx; Y. Yang, None; P. Roul, None; J. Rojas, None; B. Sauer, None; G. Cannon, None; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.

