Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1150–1165) Spondyloarthritis Including PsA – Basic Science Poster
1153: Differentially Expressed Genes in Males and Females with Ankylosing Spondylitis (AS): Transcriptomics of IL-17-producing Cells in Patients with AS
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CL
Chan Mi Lee, MD, PhD
University Hospitals
Cleveland, OH, United States
Abstract Poster Presenter(s)
Chan Mi Lee1, Ricky Chan2, Maricela Haghiac3 and Marina Magrey4, 1University Hospitals, Cleveland, OH, 2Case Western Reserve University, Cleveland, OH, 3Metrohealth Medical Center, Cleveland, OH, 4Case Western Reserve University, University Hospitals, Richfield, OH
Background/Purpose: Unlike most autoimmune diseases, ankylosing spondylitis (AS) is more frequently diagnosed in men (3:1) and with a higher radiographic progression compared to women. Th17 cells have been recognized as key players in AS pathogenesis, and men have a higher Th17 repertoire compared to women. To better understand what other immune pathways are implicated in AS pathogenesis driving this difference, we compared the transcriptome of IL-17-stimulated peripheral blood mononuclear cells (PBMCs) in male and female AS patients.
Methods: Peripheral blood samples (30mL) were obtained from 10 female and 11 male subjects ≥18 years of age with AS based on modified New York diagnostic criteria at the Clinical Research Unit of MetroHealth Medical Center per approved IRB protocol. Isolated PBMCs were stimulated with CytoStim for 4 hrs and processed through IL-17 secretion enrichment kit (Miltenyi Biotec). IL-17-positive cells were counted, and total RNA was extracted using Takara kit (Takara Biotech). Next generation RNA-sequencing (RNA-seq) was performed at Novogene. The data were analyzed using Advaita Bio's iPathwayGuide, and relative fold (logFC) was calculated using female AS patients as baseline. Genes were identified using a threshold of 0.05 for statistical significance and with absolute log fold change of at least 0.6. Proinflammatory cytokines and genes involved in Th17 differentiation, signaling, and function were identified and assessed based on prior Th17 transcriptome studies using human cells.
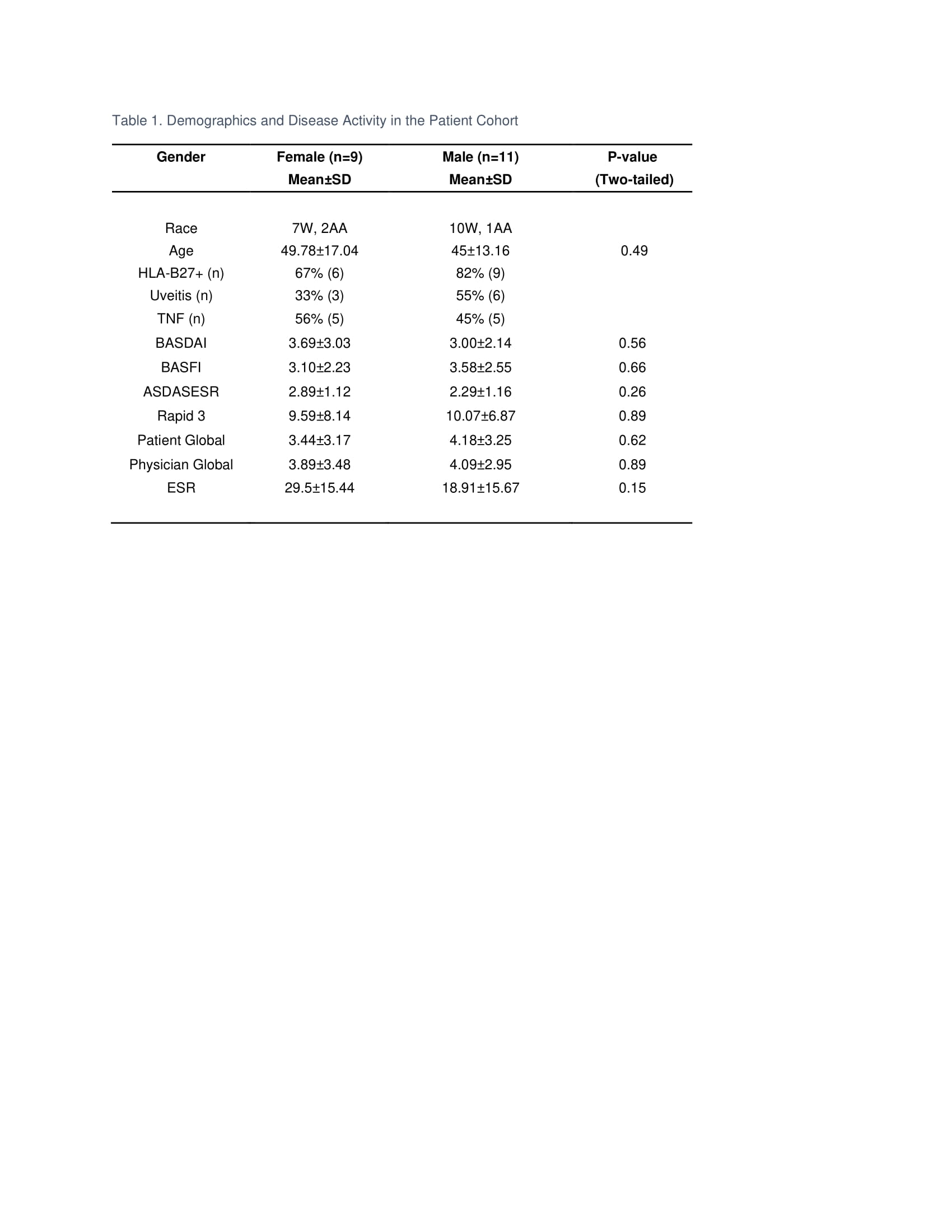
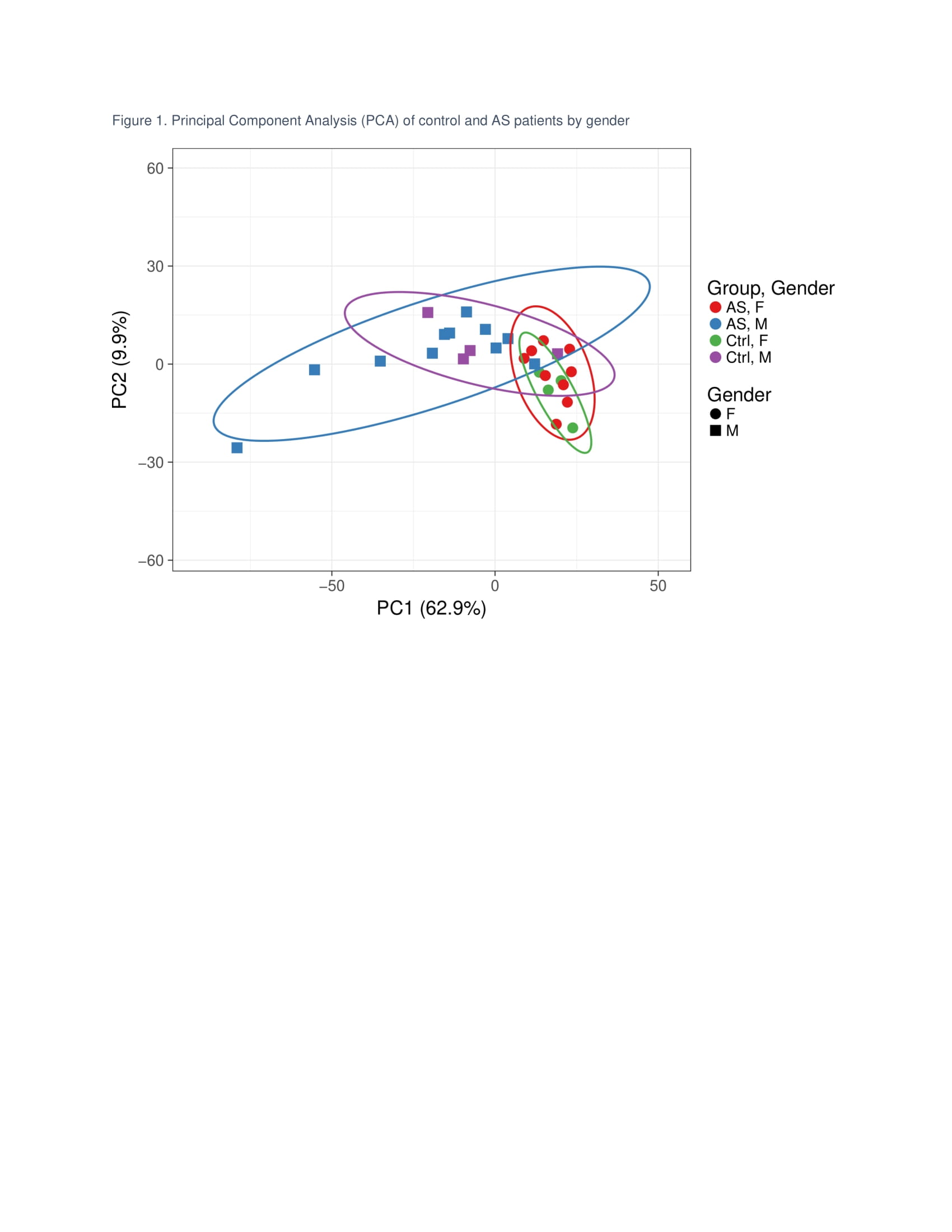
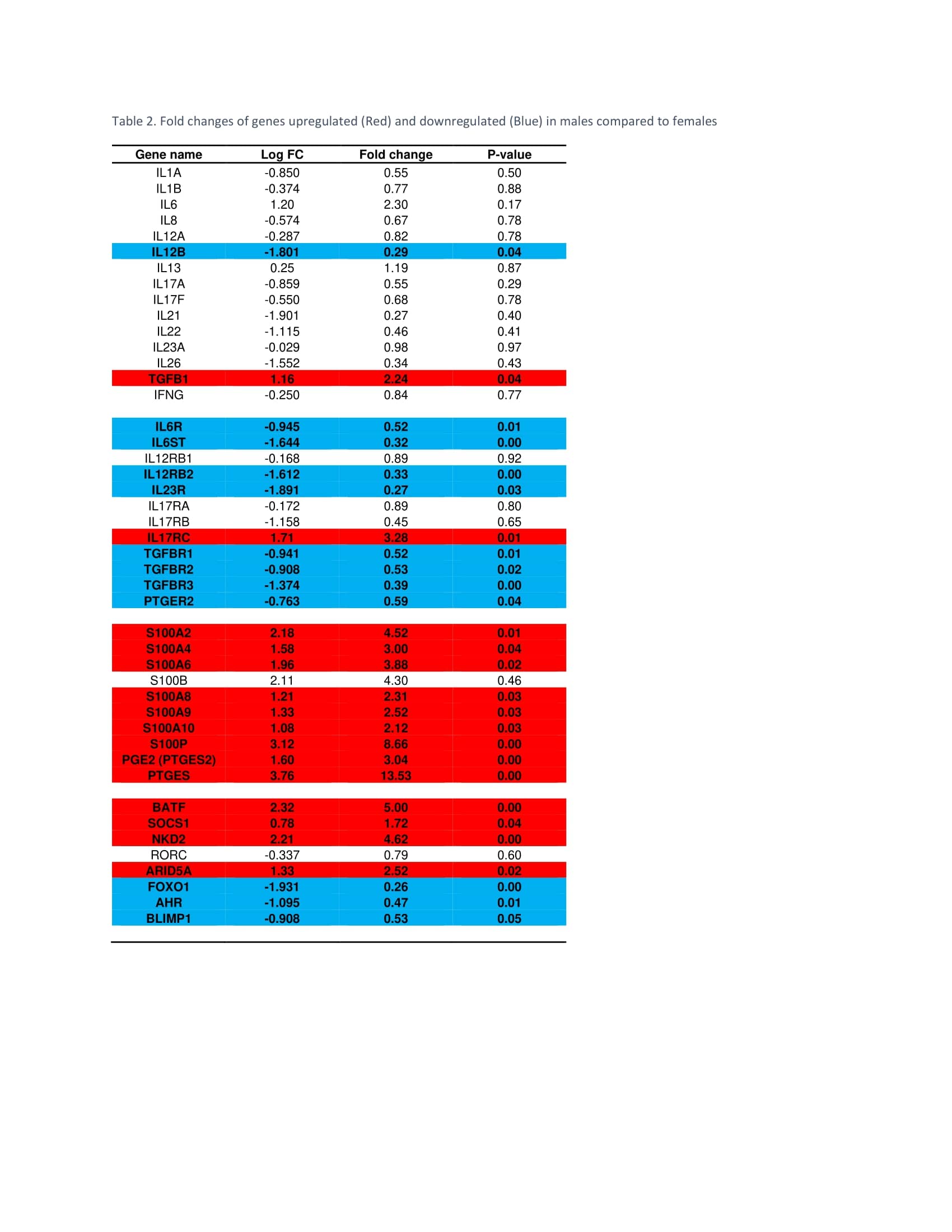
Results: The age and disease activity of patients in each gender group were comparable (Table 1). RNA-seq identified 12,893 genes with 2,851 genes with P-values < 0.05. The Principal Component Analysis (PCA; Figure 1) showed distinct patterns of gene expression in male versus female AS. Looking at Th17 inducers, there was no difference in IL-1, 6, 8, 13, 17, 21, 22, 23, or 26, but lower levels of IL-12B in males. Interestingly, receptors of IL-6, 12, 23, TGF-β and PGE2 were down-regulated in males compared to females except for IL-17RC, which was upregulated. Notably, TGF-β, PGE2, and S100 proteins including S100A2, 4, 6, 8, 9, 10, and S100P were highly upregulated in males. Genes involved in Th17 differentiation, notably BATF, SOCS1, NKD2, and ARID5A were elevated in men, while FOXO1, which inhibits RORC, was decreased. Interestingly, genes involved in the differentiation of type 1 regulatory T cells (Tr1) such as AHR and BLIMP1 were significantly downregulated in men (Table 2).
Conclusion: In-depth transcriptomic analysis of the IL-17 producing cells in male versus female AS patients revealed that, consistent with prior findings, males may have a higher propensity to produce Th17 cells by higher levels of Th17-associated transcription factors. Radiographic progression in men may be mediated by S100 proteins and prostaglandins. These findings need to be confirmed by RT-PCR and in larger studies.
Disclosures: C. Lee, None; R. Chan, None; M. Haghiac, None; M. Magrey, AbbVie, Eli Lilly, Novartis, Pfizer Inc, UCB.
Background/Purpose: Unlike most autoimmune diseases, ankylosing spondylitis (AS) is more frequently diagnosed in men (3:1) and with a higher radiographic progression compared to women. Th17 cells have been recognized as key players in AS pathogenesis, and men have a higher Th17 repertoire compared to women. To better understand what other immune pathways are implicated in AS pathogenesis driving this difference, we compared the transcriptome of IL-17-stimulated peripheral blood mononuclear cells (PBMCs) in male and female AS patients.
Methods: Peripheral blood samples (30mL) were obtained from 10 female and 11 male subjects ≥18 years of age with AS based on modified New York diagnostic criteria at the Clinical Research Unit of MetroHealth Medical Center per approved IRB protocol. Isolated PBMCs were stimulated with CytoStim for 4 hrs and processed through IL-17 secretion enrichment kit (Miltenyi Biotec). IL-17-positive cells were counted, and total RNA was extracted using Takara kit (Takara Biotech). Next generation RNA-sequencing (RNA-seq) was performed at Novogene. The data were analyzed using Advaita Bio's iPathwayGuide, and relative fold (logFC) was calculated using female AS patients as baseline. Genes were identified using a threshold of 0.05 for statistical significance and with absolute log fold change of at least 0.6. Proinflammatory cytokines and genes involved in Th17 differentiation, signaling, and function were identified and assessed based on prior Th17 transcriptome studies using human cells.
Results: The age and disease activity of patients in each gender group were comparable (Table 1). RNA-seq identified 12,893 genes with 2,851 genes with P-values < 0.05. The Principal Component Analysis (PCA; Figure 1) showed distinct patterns of gene expression in male versus female AS. Looking at Th17 inducers, there was no difference in IL-1, 6, 8, 13, 17, 21, 22, 23, or 26, but lower levels of IL-12B in males. Interestingly, receptors of IL-6, 12, 23, TGF-β and PGE2 were down-regulated in males compared to females except for IL-17RC, which was upregulated. Notably, TGF-β, PGE2, and S100 proteins including S100A2, 4, 6, 8, 9, 10, and S100P were highly upregulated in males. Genes involved in Th17 differentiation, notably BATF, SOCS1, NKD2, and ARID5A were elevated in men, while FOXO1, which inhibits RORC, was decreased. Interestingly, genes involved in the differentiation of type 1 regulatory T cells (Tr1) such as AHR and BLIMP1 were significantly downregulated in men (Table 2).
Conclusion: In-depth transcriptomic analysis of the IL-17 producing cells in male versus female AS patients revealed that, consistent with prior findings, males may have a higher propensity to produce Th17 cells by higher levels of Th17-associated transcription factors. Radiographic progression in men may be mediated by S100 proteins and prostaglandins. These findings need to be confirmed by RT-PCR and in larger studies.



Disclosures: C. Lee, None; R. Chan, None; M. Haghiac, None; M. Magrey, AbbVie, Eli Lilly, Novartis, Pfizer Inc, UCB.

