Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1495: How Does Body Mass Index Affect Secukinumab Treatment Outcomes and Safety in Patients with Ankylosing Spondylitis? – Real World Data from a German Observational Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- UK
Uta Kiltz, PhD
Rheumazentrum Ruhrgebiet
D-44649 Herne, Germany
Abstract Poster Presenter(s)
Uta Kiltz1, Jan Brandt-Juergens2, Peter Kästner3, Elke Riechers4, Daniel Peterlik5, Christina Budden5 and Hans-Peter Tony6, 1Rheumazentrum Ruhrgebiet, Herne, Germany, 2Rheumatologische Schwerpunktpraxis, Berlin, Germany, 3Ambulantes Rheumazentrum, Medizinisches Versorgungszentrum, Erfurt, Germany, 4Medizinische Hochschule Hannover, Hannover, Germany, 5Novartis Pharma GmbH, Nürnberg, Germany, 6Medizinische Klinik II - Rheumatologie/Immunologie, Universitätsklinikum Würzburg, Würzburg, Germany
Background/Purpose: Obesity is a risk factor for worse overall health in people with ankylosing spondylitis (AS)1. The German non-interventional study AQUILA provides real-world data in AS on the influence of body mass index (BMI) on therapeutic effectiveness and safety under treatment with secukinumab, a fully human monoclonal antibody that selectively inhibits IL-17A.
The aims of this interim analysis are to describe selected baseline (BL) demographics and to evaluate secukinumab treatment outcomes on disease activity and global functioning and health and to report safety profile depending on the BMI of AS patients (pts).
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 pts with active AS or psoriatic arthritis. Pts were observed from BL up to week (w) 52 according to clinical routine. Real-world data were assessed prospectively and analyzed as observed. Validated questionnaires were used to collect data on disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI) and global functioning and health (Assessment of SpondyloArthritis-Health Index, ASAS-HI). For calculation of proportion of pts that experienced (serious) adverse events ((S)AEs), all AS pts were included that received at least one dose of secukinumab. This interim analysis focuses on BMI subgroups ≤25 kg/m2 (normal weight), ˃25 to ≤30 kg/m2 (overweight) and ˃30 kg/m2 (obese) in AS pts.
Results: At BL, BMI data were available for 667 AS pts: 33.6% (n=224) normal weight, 39.9% (n=266) overweight and 26.5% (n=177) obese AS pts (Table 1). In all BMI subgroups the proportion of men was higher, even doubled among overweight AS pts. As BMI increased, so did age and comorbidities/extraarticular manifestations (EAMs, Table 1). Mean BASDAI developed similarly over time with lowest scores for normal weight and highest scores for obese AS pts (Figure 1A). Mean improvement from BL to w52 was 1.3 (27.1%) for normal weight, 1.5 (27.2%) for overweight, and 1.2 (21.8%) for obese AS pts.
Mean ASAS-HI at BL was similar for all BMI subgroups (≤25: 7.4; ˃25-≤30: 7.7; >30: 8.1); the best improvement was observed in normal weight, the least in obese AS pts (Figure 1B). Mean improvement from BL to w52 was 2.1 (28.4%) for normal weight, 1.3 (16.9%) for overweight, and 0.6 (7.4%) for obese AS pts. The occurrence of AEs/SAEs with or without suspected relationship to secukinumab increased with increasing BMI. For example, the percentage of SAEs in normal weight was 21%, in overweight 26.7% and in obese AS pts 30.9% (data not shown). There were no events with fatal outcome or unexpected safety signals in either subgroup.
Conclusion: In a real-world setting, secukinumab improved disease activity and global functioning and health in all BMI subgroups of AS pts; normal weight AS pts had numerically better ASAS-HI and BASDAI scores than obese AS pts. Altogether, real-world data of this interim analysis show that secukinumab is an effective treatment with a favorable safety profile up to 52 weeks in AS pts in all BMI subgroups.
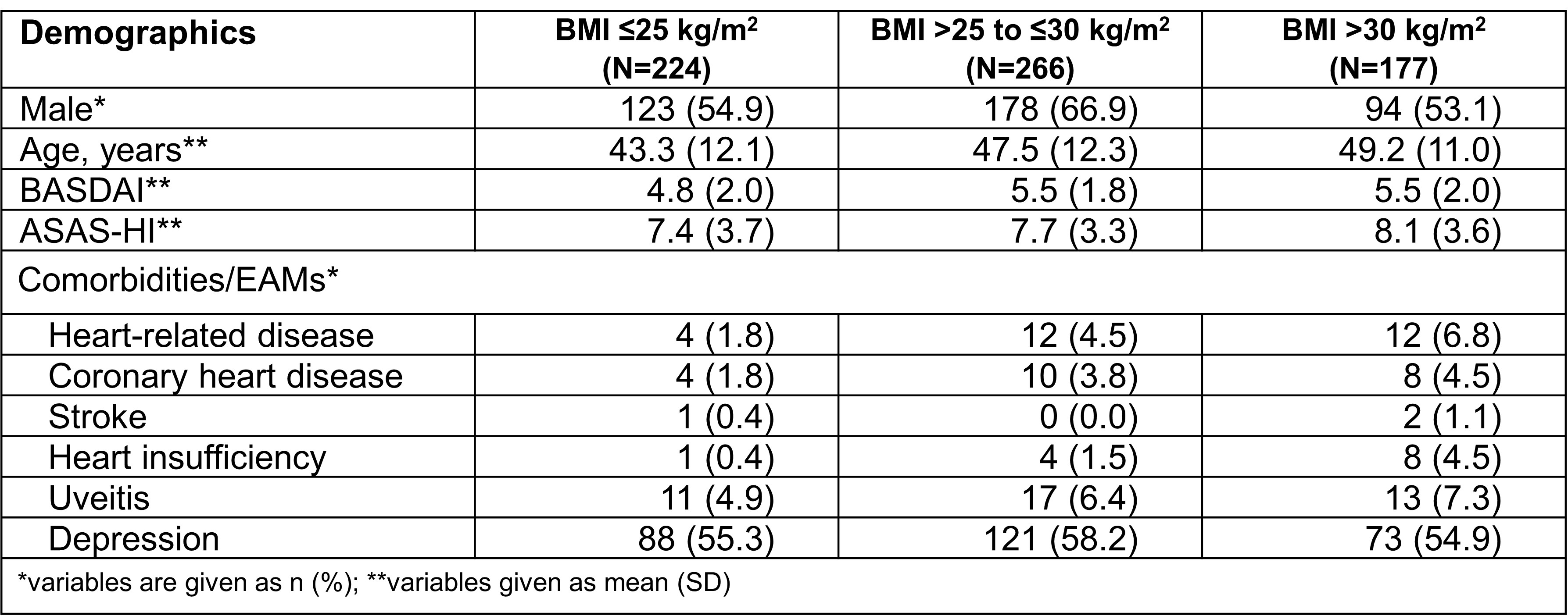 Table 1: Overview of baseline characteristics in AS pts depending on BMI
Table 1: Overview of baseline characteristics in AS pts depending on BMI
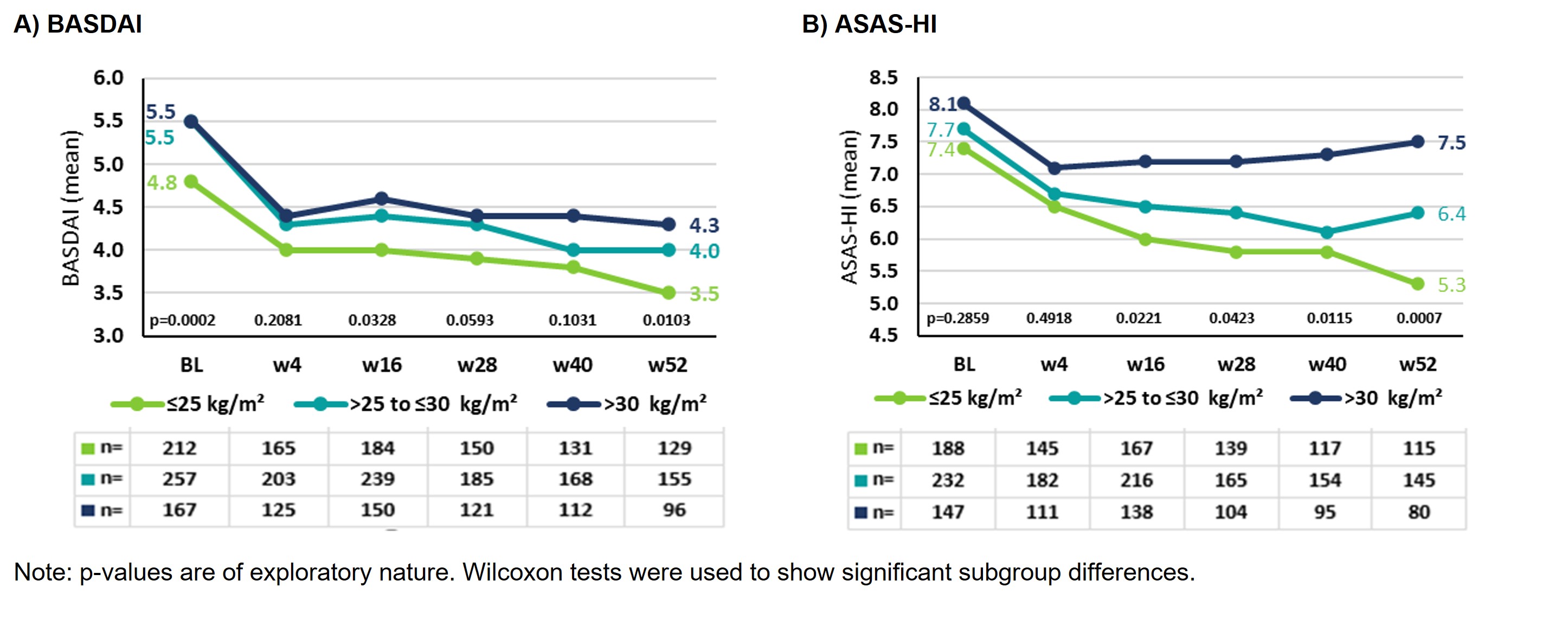 Figure 1: Disease activity and global functioning under secukinumab treatment in AS pts stratified by BMI
Figure 1: Disease activity and global functioning under secukinumab treatment in AS pts stratified by BMI
Disclosures: U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; P. Kästner, Chugai, Novartis; E. Riechers, AbbVie, Chugai, Lilly, Janssen, Novartis, Pfizer, Roche, UCB; D. Peterlik, Novartis; C. Budden, Novartis; H. Tony, AbbVie, Astra-Zeneca, BMS, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi.
Background/Purpose: Obesity is a risk factor for worse overall health in people with ankylosing spondylitis (AS)1. The German non-interventional study AQUILA provides real-world data in AS on the influence of body mass index (BMI) on therapeutic effectiveness and safety under treatment with secukinumab, a fully human monoclonal antibody that selectively inhibits IL-17A.
The aims of this interim analysis are to describe selected baseline (BL) demographics and to evaluate secukinumab treatment outcomes on disease activity and global functioning and health and to report safety profile depending on the BMI of AS patients (pts).
Methods: AQUILA is an ongoing, multi-center, non-interventional study including up to 3000 pts with active AS or psoriatic arthritis. Pts were observed from BL up to week (w) 52 according to clinical routine. Real-world data were assessed prospectively and analyzed as observed. Validated questionnaires were used to collect data on disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI) and global functioning and health (Assessment of SpondyloArthritis-Health Index, ASAS-HI). For calculation of proportion of pts that experienced (serious) adverse events ((S)AEs), all AS pts were included that received at least one dose of secukinumab. This interim analysis focuses on BMI subgroups ≤25 kg/m2 (normal weight), ˃25 to ≤30 kg/m2 (overweight) and ˃30 kg/m2 (obese) in AS pts.
Results: At BL, BMI data were available for 667 AS pts: 33.6% (n=224) normal weight, 39.9% (n=266) overweight and 26.5% (n=177) obese AS pts (Table 1). In all BMI subgroups the proportion of men was higher, even doubled among overweight AS pts. As BMI increased, so did age and comorbidities/extraarticular manifestations (EAMs, Table 1). Mean BASDAI developed similarly over time with lowest scores for normal weight and highest scores for obese AS pts (Figure 1A). Mean improvement from BL to w52 was 1.3 (27.1%) for normal weight, 1.5 (27.2%) for overweight, and 1.2 (21.8%) for obese AS pts.
Mean ASAS-HI at BL was similar for all BMI subgroups (≤25: 7.4; ˃25-≤30: 7.7; >30: 8.1); the best improvement was observed in normal weight, the least in obese AS pts (Figure 1B). Mean improvement from BL to w52 was 2.1 (28.4%) for normal weight, 1.3 (16.9%) for overweight, and 0.6 (7.4%) for obese AS pts. The occurrence of AEs/SAEs with or without suspected relationship to secukinumab increased with increasing BMI. For example, the percentage of SAEs in normal weight was 21%, in overweight 26.7% and in obese AS pts 30.9% (data not shown). There were no events with fatal outcome or unexpected safety signals in either subgroup.
Conclusion: In a real-world setting, secukinumab improved disease activity and global functioning and health in all BMI subgroups of AS pts; normal weight AS pts had numerically better ASAS-HI and BASDAI scores than obese AS pts. Altogether, real-world data of this interim analysis show that secukinumab is an effective treatment with a favorable safety profile up to 52 weeks in AS pts in all BMI subgroups.
 Table 1: Overview of baseline characteristics in AS pts depending on BMI
Table 1: Overview of baseline characteristics in AS pts depending on BMI Figure 1: Disease activity and global functioning under secukinumab treatment in AS pts stratified by BMI
Figure 1: Disease activity and global functioning under secukinumab treatment in AS pts stratified by BMIDisclosures: U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; P. Kästner, Chugai, Novartis; E. Riechers, AbbVie, Chugai, Lilly, Janssen, Novartis, Pfizer, Roche, UCB; D. Peterlik, Novartis; C. Budden, Novartis; H. Tony, AbbVie, Astra-Zeneca, BMS, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi.

